Lavinia Woodward, Senior Product Development Producer at Beacon, shared a post on LinkedIn:
“TOPO1i is today’s favourite ADC payload mechanism, but what will be tomorrow’s?
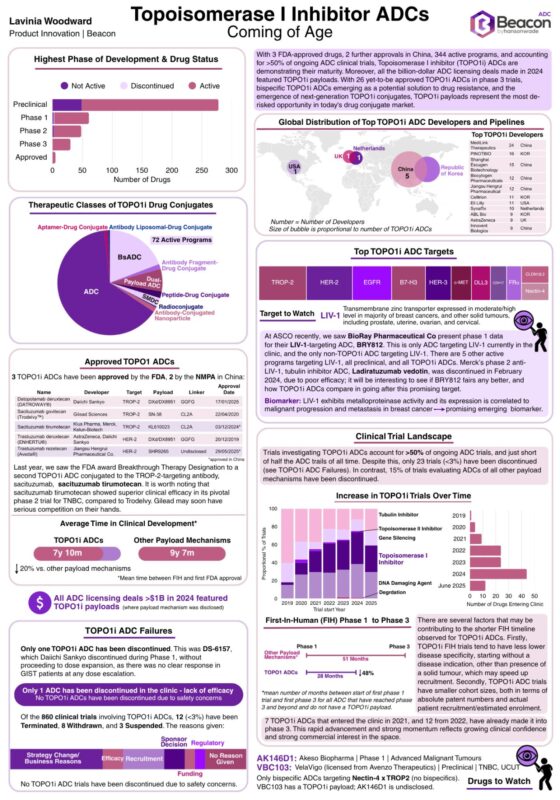
The data is in, and it’s decisive: the momentum behind Topoisomerase I inhibitor (TOPO1i) ADCs has accelerated into 2025. Is this justified, or are we seeing ‘me too’ developers jumping on the TOPO1i bandwagon? Let me know your thoughts in the comments.
Market Validation
- Enhertu leads 2024 ADC sales at $3.8B, Trodelvy at $1.3B.
- All the $1B+ ADC licensing deals in 2024 (where payload mechanism disclosed) = TOPO1i ADCs.
- Nearly 800 ongoing trials, and <3% clinical discontinuation rate.
Innovation Pipeline
- Faster clinical development timelines vs other payload classes.
- 72 bispecific TOPO1i ADCs in active development (4 in Phase 3). Include novel Nectin-4 x TROP-2 target pair.
- While DXd continues to be the dominant payload, use of diverse scaffolds is indicative of innovation and opportunity for significant refinement within the Topo1i ADC class.
- After failure of Merck Life Science’s tubulin inhibitor ADC, Ladiratuzumab vedotin, TOPO1i ADCs are going after LIV-1. A target to watch, for sure.
Competition Intensifies
- 5 of the top 10 TOPO1i developers are headquartered in China. Further 3 have headquarters in the Republic of Korea. The geographic diversification of expertise is accelerating platform evolution.
- Last year, the FDA awarded Breakthrough Therapy Designation to sacituzumab tirumotecan, a second TOPO1i ADC using the TROP-2 targeting antibody, sacituzumab. Sacituzumab tirumotecan showed superior efficacy in pivotal Phase 2 TNBC trial, than seen with Trodelvy. Gilead Sciences may soon face serious competition.
Key Takeaway: With proven commercial success, robust pipelines, and expanding global competition, this payload mechanism represents both immediate opportunity and long-term potential.
What’s your take on these developments?
- If (when) approved in the US, will sacituzumab tirumotecan’s superiority disrupt Trodelvy’s $1.3B sales?
- Will bispecific TOPO1i ADCs solve the resistance challenge?
- TOPO1i is today’s favourite payload mechanism, but what will be tomorrow’s? Degraders? Immunostimulants?
Analysis done using data from the Beacon ADC database.”
Paolo Tarantino, 2025 Yvonne’s “Top Voice” Award Winner, Research Fellow at Dana-Farber Cancer Institute, shared this post, adding:
“My take:
- if no Topo1 ADC is yet approved for a certain oncology indication, there will most likely be one approved by 2030
- if one or more Topo1 ADCs are already approved (e.g. breast), the path will be much more challenging. Cross-resistance between Topo1 ADCs used in sequence — even with different targets — is real.”
More posts featuring TOPO1i.


