Giannis Mountzios, Director of the 4th Oncology Department and Clinical Trials Unit at Henry Dunant Hospital Center, posted on X:
“3 weeks after ASCO25, let’s digest the data from the landmark DeLLphi-304 trial published in NEJM that changed the >30 years SoC in recurrent SCLC from immunobiology and clinical standpoints.
The field of recurrent SCLC has been dominated for more that 3 decades by monochemotherapy with Topo1-inhibitors/taxanes and recently lurbinectedin and amrubicin with modest outcomes and mOS of 6-8 months.
Immunotherapy should be active in SCLC (high TMB, smoking related gene signatures), but the addition of ICIs to chemo in 1L offers modest net benefit in mOS of approximately 2 months in pos+trials.
In brief, two major facts drive SCLC transformation: Knock-out of the two main tumor suppressor genes (TP53, RB1) leading to uncontrolled proliferation and NOTCH deregulation that triggers neuroendocrine factor transcription, leading to EMT and early chemoresistance.
Discovery of molecular SCLC subtypes led to recognition of “ more neuroendocrine” phenotypes (ASCL1, YAP, NEUROD1) and “less neuroendocrine” phenotypes (POU2F3) and the famous “inflammatory” phenotype characterized by intact NOTCH signaling and better outcomes.
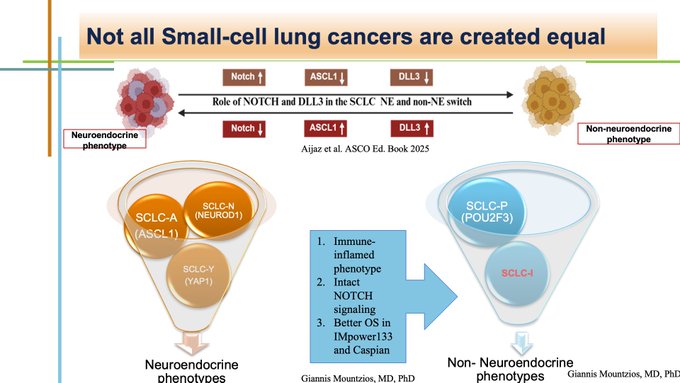
Works from many groups showed that ASCL1 relates to DLL3 to mediate neuroendocrine transcription. DLL3 negatively regulates NOTCH signaling, hence targeting DLL3 may restore NOTCH-mediated reduction of neuroendocrine features and lead to a more “inflamed” biophenotype
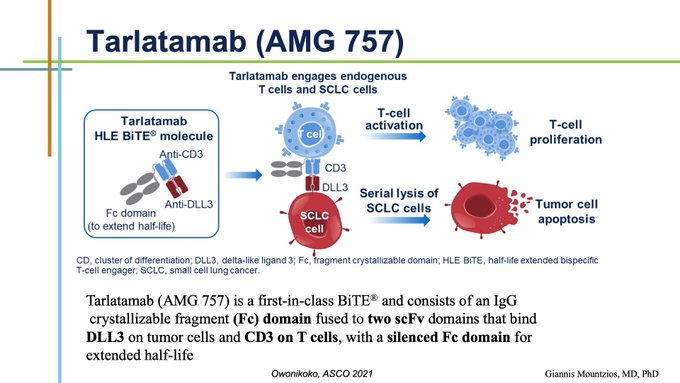
This notion gave birth to Tarlatamab, the 1st in class BiTE, a bispecific T-cell engager binding DLL3 and TCR (CD3) on the surface of CD4/8+ T-lymphocytes, triggering SCLC cell lysis and apoptosis (picture from Taofeek K Owonikoko from ASCO 2021)
DeLLphi-304 was the 1st global ph3 RCT to directly compare Tarlatamab with SoC monochemotherapy (Topotecan/Lurbinectedin/Amrubicin) depending on local approval and practice, with mOS as primary outcome and PFS/PROs and key secondary endpoints.
In ITT, tarlatamab significantly improved mOS from 8.3 to 13.6 months (HR=0.6), PFS from 2.7 to 3.5 months and ORR from 20% to 35%, with consistent efficacy across all main subgroups of interest.
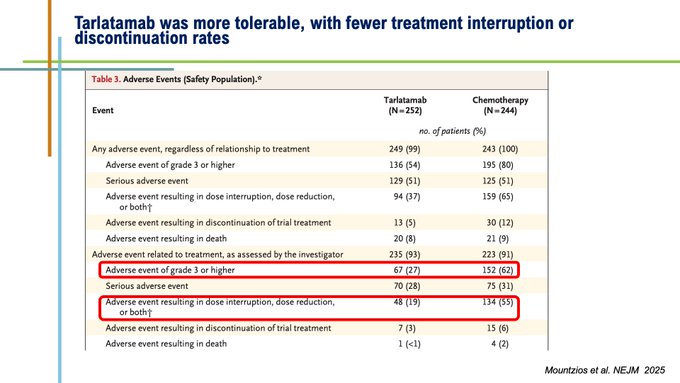
Importantly, tarlatmab was better tolerated, with fewer Grade 3/4 TRAEs and fewer interruption and dicontinuation rates. Cumbersome symptoms including dyspnoea, cough and chest pain were significantly or numerically improved.
CRS is inherently related to this class of agents. 56% of patients experienced CRS, usually in C1D1/C1D8 mainly Grade 1 or 2 (pyrexia, mild hypoxia-hypotension) which were manageable if monitored timely and treated properly. No Grade 4/5 CRS event was reported.
Immune cell effector-associated neurotoxicity syndrome (ICANS) is a less well understood entity related to cytokine disruption of BBB and comprising diverse neurological symptoms. ICANS was rare (<10%) and no ICANS 3 or more was observed. (pic from Sands Cancer 2024).
Tarlatamab is currently evaluated in 1L (DeLLphi-312), maintenance (305) and 2L with B7-H3 ADC (310). Many new BiTEs, Tri-specific (TriTEs) and BiTE ADCs are under clinical development as monotherapy or combos with ICIs or chemo (picture from Aijaz et al. ASCO Ed Book 2025).”
More posts featuring Giannis Mountzios.


