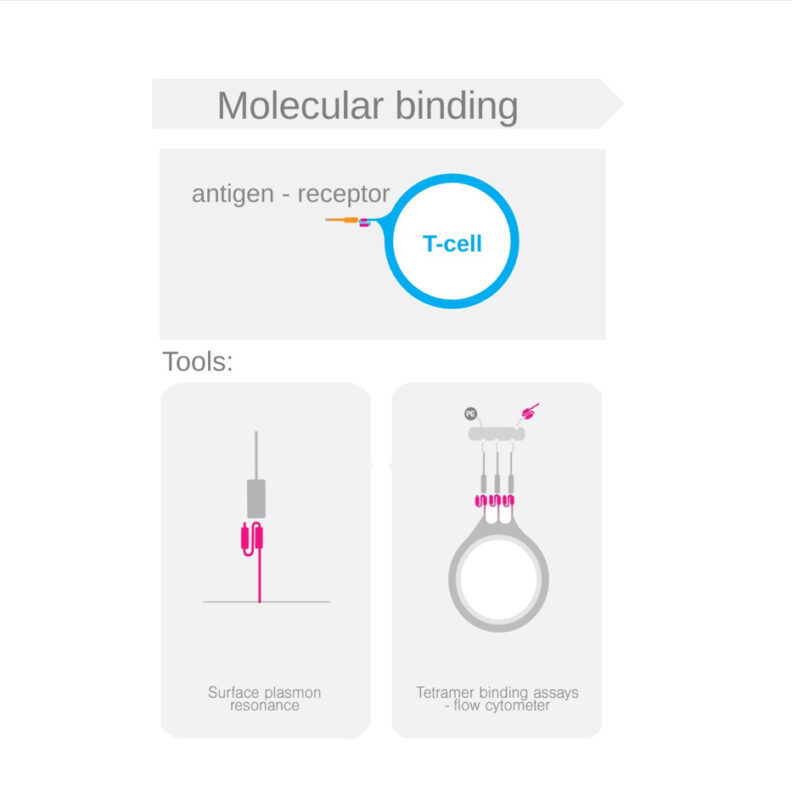
Julian Ashby, Product Marketing Manager at LUMICKS, shared on LinkedIn:
“Each year, companies use tens of thousands of animals for immuno-oncology drug testing.
Yet, more than nine in ten drugs that enter human clinical trials fail because they are unsafe or ineffective.
This has led to the FDA no longer requiring animal tests before human drug trials, paving the way for deep characterization using preclinical in vitro assays.
However, these assays alone provide insufficient detail for evaluating cell therapies.