Oncology Brothers made the following post on X:
“Nivo/Gem/Cis now FDA Oncology approved for 1L metastatic bladder cancer based on CM901:
- Improved OS w/ Nivo (HR 0.78) 21.7mos Vs 18.9mos
- ≥ Gr3 AEs in 62% Vs 52%
- New Option (if a patient is not a candidate for EV302)”
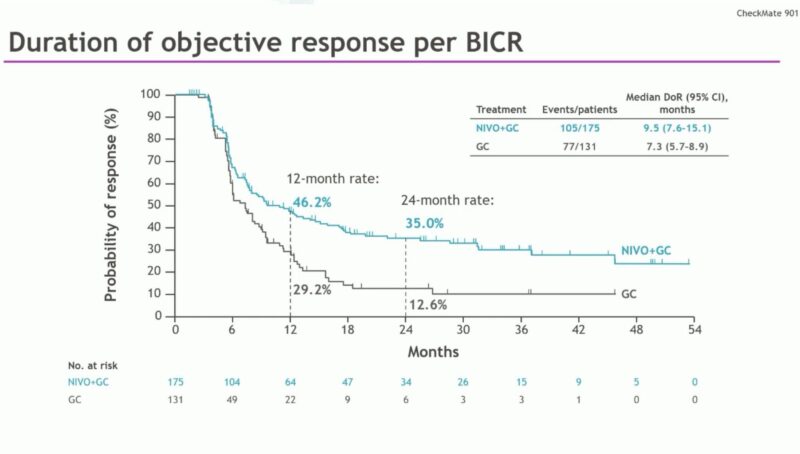
Source: Oncology Brothers/X


