Talha Badar, Hematologist/Oncologist at Mayo Clinic in Jacksonville, Florida, shared a thread on X/Twitter:
“Weekend review: Sub-group of Acute Myeloid Leukemia (AML) with targetable mutation have sub-optimal response of Venetoclax (VEN) with Hypomethylating agents (HMA), for example, FLT3m, KMT2A OR sequencing targeted therapy post-VEN have modest responses, for example, IDH1/2i.
Brief review focusing on VEN+HMA triplets in AML:
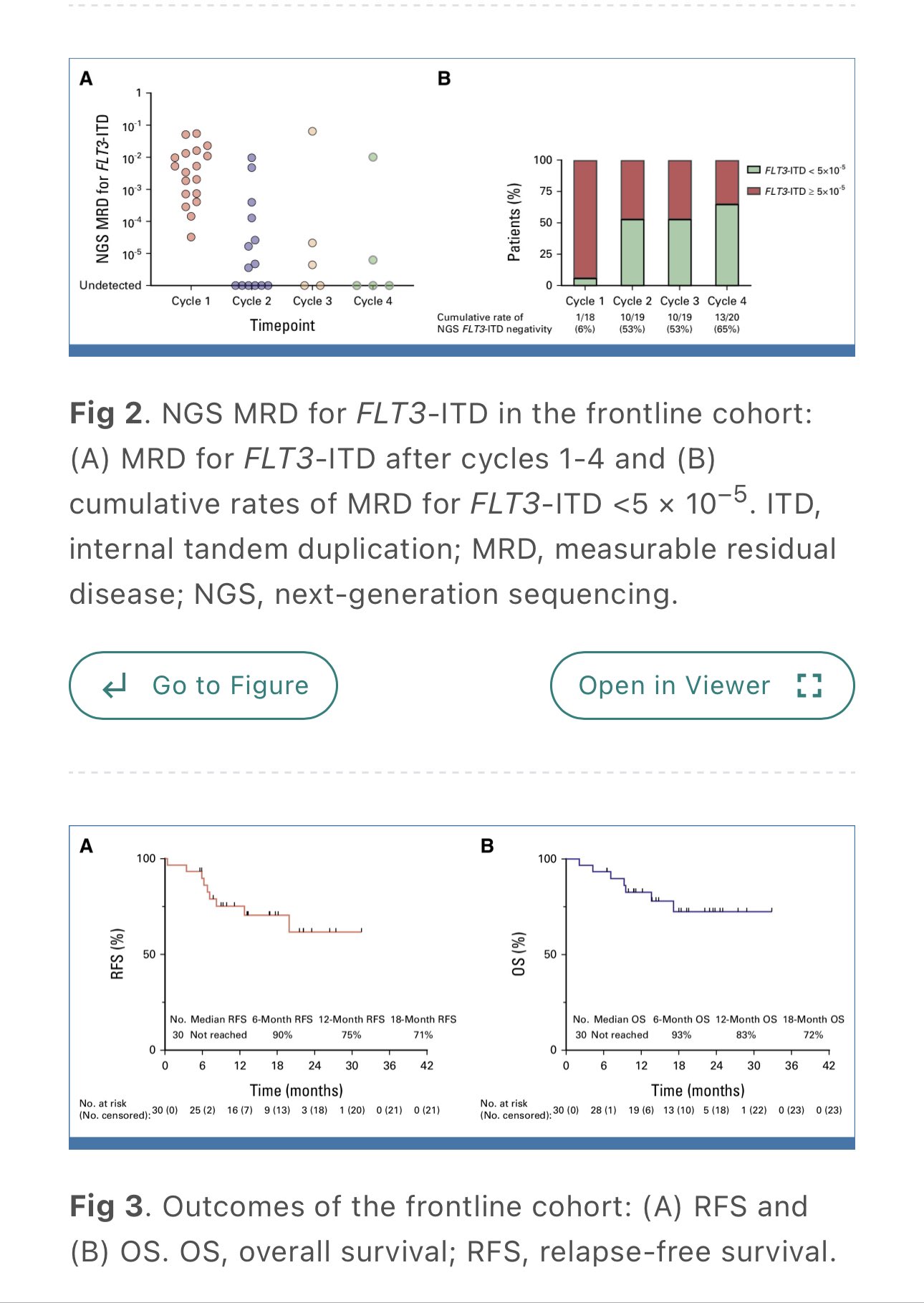
- Phase I/II VEN+AZA with Gilt (MTD; 80 mg) in ND and RR FLT3m AML
- C1 AZA 7d, VEN 28d, Gilt 28d
- VEN and Gilt held if d14 BM hypoplastic/ <5% blast
- C2 AZA 5d, VEN 7d, Gilt 28d
- 30 ND, CR/CRi 96% 18 mo RFS/OS 71/72%. 2
- 2 RR, CR/CRi 27%
- VEN (14-21d), DEC (10d) and Quizartinib (30-40mg daily);
- 28 pts (23 RR AML)
- RR gp CRc 78%, mOS 7.8 mo
- ND gp CRc 100%, mOS 19/8 mo with/without alloHCT.
- G3/4 non-hem AE 30-40%, 60d mortality 5%
- IVO, VEN +/- AZA in IDH1m
- Phase1b/II study on 31 pts. AE were G1/2 91%
- CRc= IVO + VEN + AZA vs IVO + VEN 90%vs 83%; OS NR vs 42 mo
- Phase 1: AZA+VEN and APR246 in TP53m AML
- 49 pts No DLT, sAE 27%
- CR 38%, ORR 64%
- Aprea myeloid program was stopped after it fails to meet PE of CR in HR-MDS.
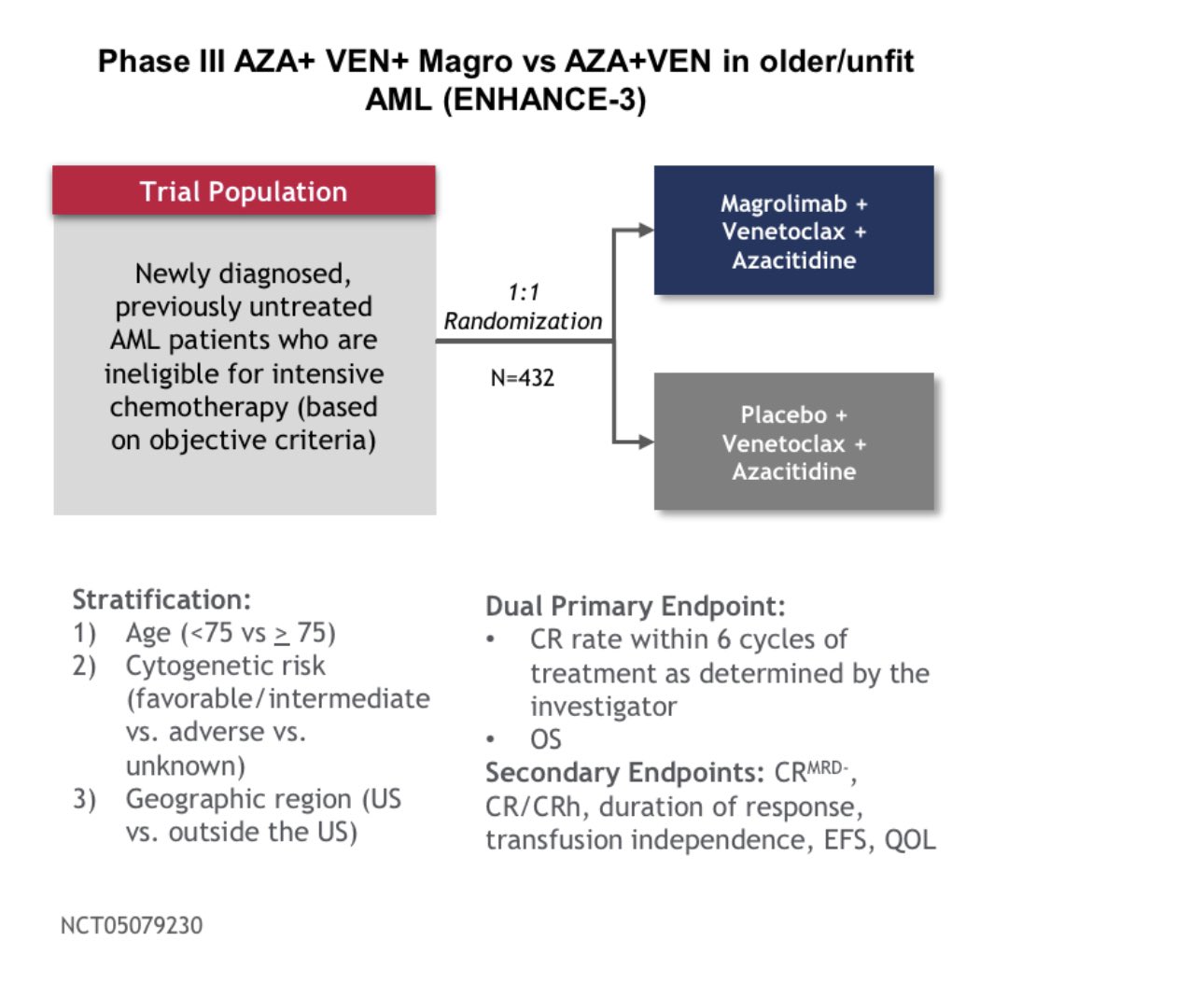
- Ph1b/II study evaluated VEN+HMA+Magro triplet in AML;
- 43 pts/ 27 TP53m: CR 63%/ 83% TP53m. 12 mo OS 53 vs 83%.
- ENHANCE-III trial comparing Ven/HMA to Ven/HMA+ Magro halted; Magro demonstrated futility and increased the risk of death
- VEN+HMA with Tagraxofusp (TAG)
- TAG 12 μg/kg/d for 3d, with 7-d AZA +/− 21-d VEN.
- Expansion cohort of 26 pts ELN adverse risk (50% TP53 mutated), ORR 69%, 39% CR, 19% CRi
- PFS 14 and mOS 8.5 mo
Summary slide: Venetoclax plus HMA triplets!
Source: Talha Badar/X