Al-Ola A Abdallah, Associate Professor and Plasma Cell disorder program Director at University of Kansas Medical Center, shared a post on X:
“Daratumumab-Based Quadruplet vs. Triplet Induction Regimens in Transplant-Eligible NDMM:
Meta-analysis:
1The analysis of four studies (3 randomized controlled trials & 1 non-randomized controlled study) involving 3327 patients demonstrates that daratumumab-based quadruplet regimens.
Daratumumab-based quadruplet versus triplet induction regimens in transplant-eligible newly diagnosed multiple myeloma: a systematic review and meta-analysis.
Authors: João Tadeu Damian Souto Filho, et al.

The meta-analysis included four studies with a total of 3327 TE-NDMM patients.
- OS: Daratumumab-based quadruplet regimens demonstrated a statistically significant improvement in OS compared to triplet regimens (pooled HR 0.60; 95% CI 0.48–0.75; P < 0.00001).
- OS rate at 84 months of 82.5% for the quadruplet group vs 69.9% for the triplet group.
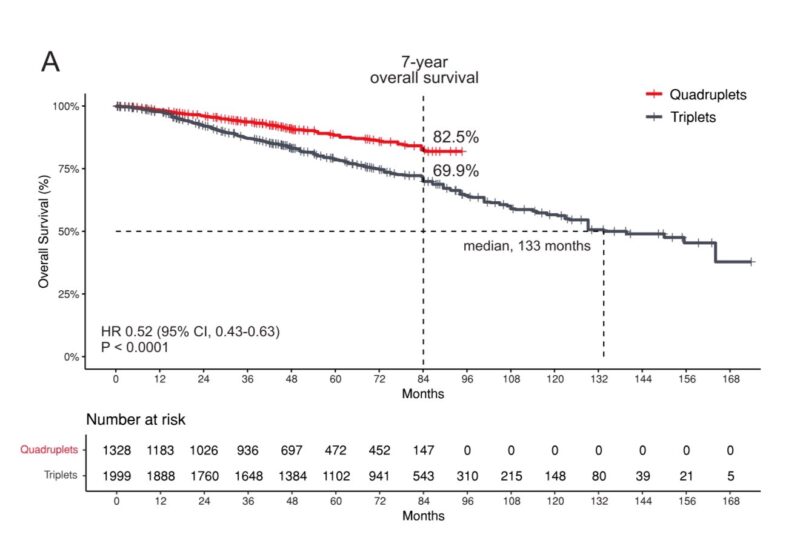
The median OS was not reached for the quadruplet group, while it was 133 months for the triplet group (HR 0.52; 95% CI, 0.43–0.63; P < 0.0001).
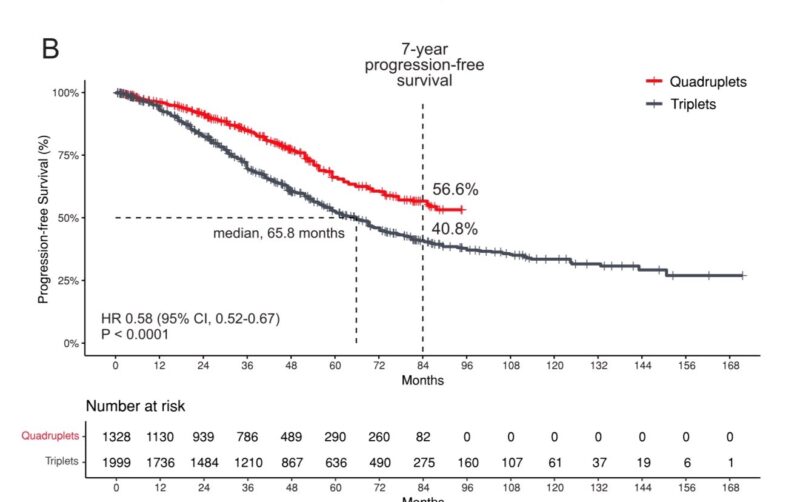
- PFS: Daratumumab-based quadruplet regimens also showed a statistically significant improvement in PFS compared to triplet regimens (pooled HR 0.49; 95% CI 0.37–0.65; P < 0.00001; I²= 52%). This represents a 51% reduction in the risk of disease progression or death.
- Estimated PFS rate at 84 months of 56.6% for the quadruplet group vs 40.8% for the triplet group.
- The median PFS was not reached for the quadruplet group, while it was 65.8 months for the triplet group (HR 0.58; 95% CI, 0.52–0.67; P < 0.0001).
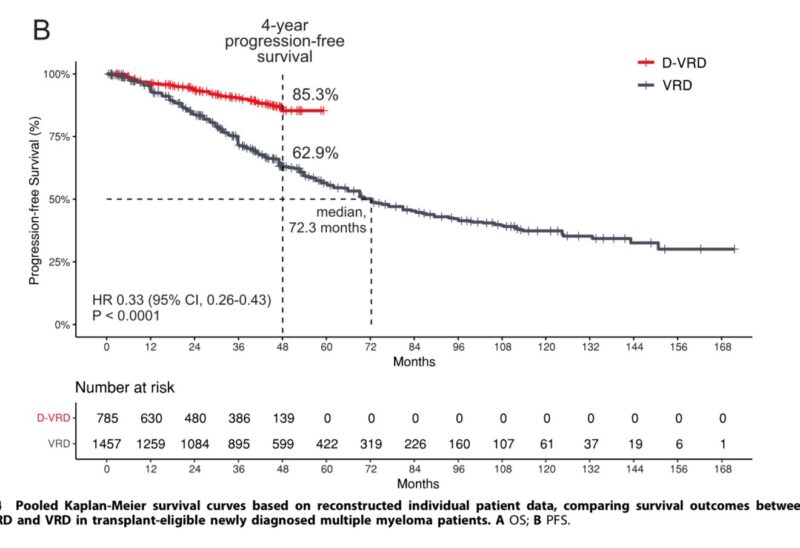
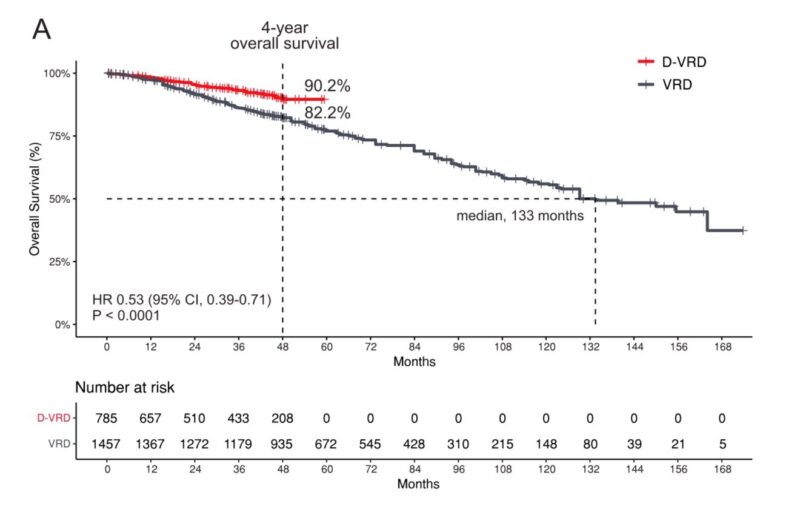
D-VRD vs. VRD Subgroup Analysis: A per-protocol subgroup analysis.
- At 48 months, the OS rate for D-VRD was 90.2% compared to 82.2% for VRD.
- Median OS was not reached for D-VRD and was 133 months for VRD (HR 0.53; 95% CI, 0.39–0.71; P < 0.0001).
- At 48 months, the PFS rate for D-VRD was 85.3% compared to 62.9% for VRD. The median PFS was not reached for D-VRD and was 72.3 months for VRD (HR 0.33; 95% CI, 0.26–0.43; P < 0.0001).
Limitations, including :
Use of aggregate data from published studies, potential heterogeneity due to variations in study designs and maintenance therapies, the influence of daratumumab use in consolidation and maintenance phases in some trials, and the limited reporting of post-protocol therapies.”


