Tom Fleischer, Co-founder & CEO at Sequentify, shared a post on LinkedIn:
“Liquid Biopsy’s Unseen Limitation.
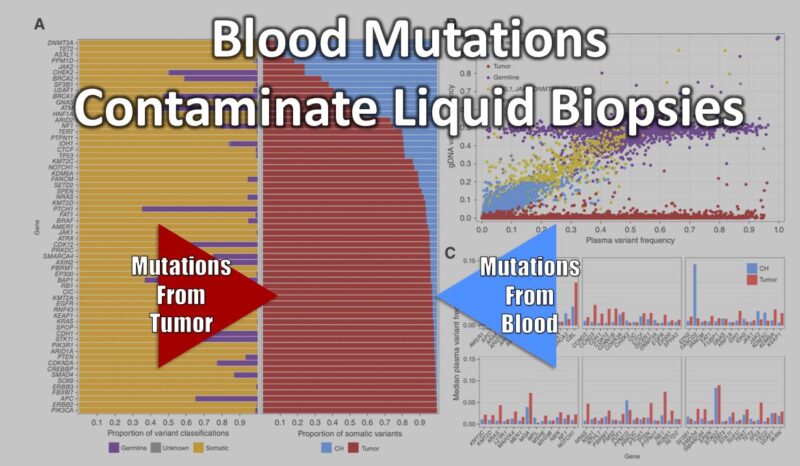
A recent study by Caris Life Sciences highlights a critical and often overlooked challenge in cfDNA testing: the impact of clonal hematopoiesis (CH). Meaning, mutations in blood cells themselves rather than in tumors.
Daniel Magee, et al. analyzed nearly 17,000 advanced cancer patients, comparing plasma cfDNA sequencing with white blood cell (WBC) sequencing. Their findings were striking:
- 42% of patients had at least one CH variant in key cancer-associated genes.
- In BRCA2, CHEK2, BRCA1, and ATM, a significant fraction of detected variants originated from blood cells, not tumors.
- CH mutations were also found in NRAS, BRAF, EGFR, and KRAS—genes widely used in therapy selection.
This issue was also flagged years ago in a Nature Medicine (2019) study by Razavi, et al. from Memorial Sloan Kettering Cancer Center, which showed that over 50% of cfDNA mutations in cancer patients were actually blood cell-derived, not tumor-related.
Why This Matters:
Without WBC sequencing, cfDNA tests can misclassify non-tumor mutations as actionable targets, leading to both false positives and false negatives, ultimately affecting treatment decisions.
- Natera (Signatera) uses matched WBC sequencing to filter out CH-related variants.
- Guardant Health (Guardant360) acknowledges CH artifacts but does not always use matched WBCs.
- Early versions of Foundation Medicine (FoundationOne Liquid) lacked explicit CH filtering, but recent updates attempt to address this.
Regulatory bodies will likely require CH filtering in the future. As more studies highlight its impact, companies that fail to account for CH risk losing credibility in precision oncology.
At Sequentify, we enable efficient and scalable cfDNA and WBC testing for labs conducting this type of analysis, which can ensure more accurate and reliable results in liquid biopsy testing.
Is your lab adjusting for CH in liquid biopsies?”
Chase G. Wasson, Vice President of Caris Life Sciences, reshared the post on LinkedIn, adding:
““They NOT like us”


