Chris De Savi, CSO at Curie.Bio, shared a post on LinkedIn:
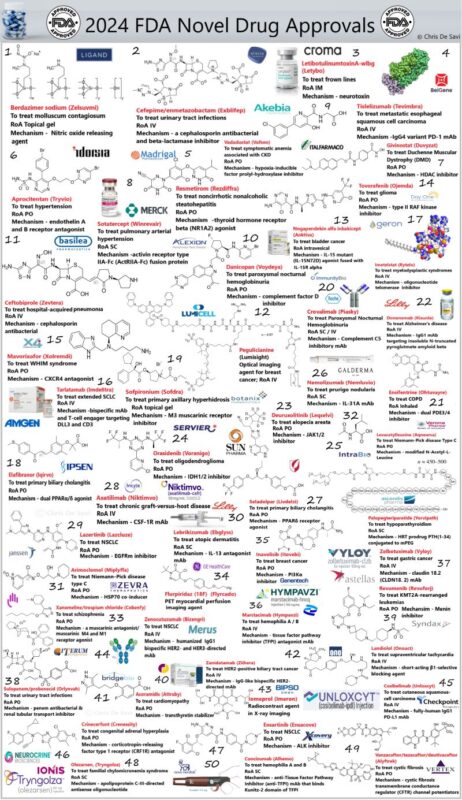
“FDA Novel Drug Approvals 2024 medicine.
That is a wrap for 2024.
And another year of relentless drug discovery.
There you have it, drug-hunting aficionados, the complete list of 2024 novel FDA-approved drugs, 50 NMEs so far, down from 2023 and up from 2022 (55 and 38 approvals, respectively).
Notable first-in-class approvals
Revumenib, a menin inhibitor that disrupts the interaction between menin and MLL proteins, halting cancer cell growth. It is indicated for relapsed or refractory acute leukemia with KMT2A translocation, providing hope to patients with resistant disease.
Zanidatamab-hrii, a bispecific antibody targeting two epitopes of the HER2 receptor, enhancing anti-tumor activity. It is approved for unresectable or metastatic HER2-positive biliary tract cancer, addressing a rare and aggressive condition with limited treatment options.
Acoramidis stabilizes the transthyretin (TTR) protein to prevent misfolding and amyloid plaque deposition. It is used for cardiomyopathy associated with transthyretin-mediated amyloidosis (ATTR-CM), improving outcomes for patients with heart failure due to amyloid deposits.
Landiolol, an ultra-short-acting beta-1 adrenergic receptor antagonist offering rapid and safe heart rate control for supraventricular tachycardia (SVT), with a reduced risk of hypotension—a significant advancement for arrhythmia management.
Crinecerfont provides a novel approach for classic congenital adrenal hyperplasia (CAH), a hormonal disorder. Acting as a corticotropin-releasing factor type 1 receptor antagonist, it reduces androgen production and restores hormonal balance.
About 40% of the approved drugs target cancer, including lung cancer, leukemia, and gastric adenocarcinoma.
Cardiovascular therapies account for 15%, focusing on conditions like transthyretin-mediated amyloidosis.
Neurological disorders account for 10%, addressing diseases such as congenital adrenal hyperplasia.
Another 10% tackle infectious diseases with new antibiotics for resistant infections.
The remaining 25% address diverse conditions, including metabolic and genetic disorders.
My drug of the year – what is yours?
Cobenfy, a groundbreaking schizophrenia treatment, combines xanomeline, a muscarinic receptor agonist, with trospium chloride, a peripheral antagonist, to uniquely target central nervous system receptors while reducing side effects. Schizophrenia, which affects 24 million globally, has seen limited innovation for decades.
By targeting muscarinic rather than dopamine pathways, Cobenfy offers improved efficacy and fewer side effects, such as weight gain and movement disorders.
As a novel class of medication, it provides a vital option for treatment-resistant patients, solidifying its status as my drug of the year.
Follow Chris De Savi or ring the bell icon to be notified of all his posts.”
For more updates, follow OncoDaily.
Chris De Savi is the Chief Scientific Officer and Partner at Curie.Bio, where he leverages his drug discovery expertise and seed investment experience to help founders launch therapeutics companies that successfully secure subsequent financing. He is also the founder of the Drug Hunting Literature Group.


