Oncology Brothers shared a post on X:
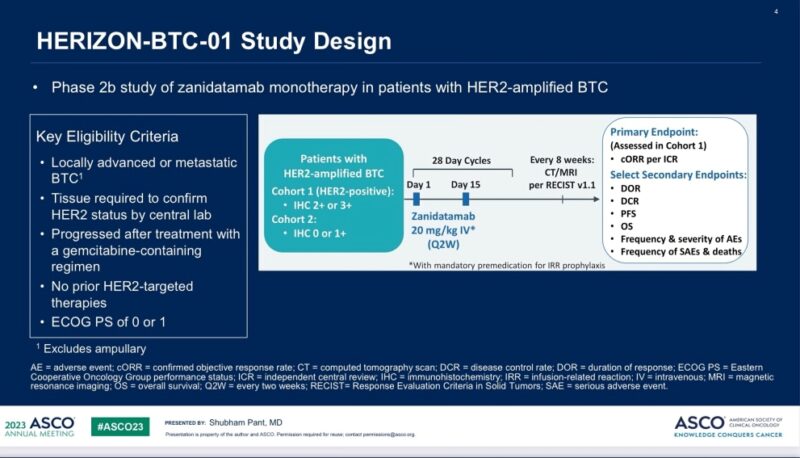
“Zanidatamab (bispecific antibody against Her2) now US FDA approved for unresectable/metastatic HER2+ biliary tract cancer based off HerizonBTC01 – Single arm Ph2B study, 20 mg/kg IV Q2W – mDoR 14.9mos – mOS 15.5mos – AEs: Diarrhea, EF.”

More posts featuring Oncology Brothers.


