Paolo Tarantino and Raffaele Colombo shared posts on X, about recent paper published in Cancer Discovery:
The Journey of Antibody–Drug Conjugates: Lessons Learned from 40 Years of Development.
Authors: Raffaele Colombo, Paolo Tarantino, Jamie R. Rich, Patricia M. LoRusso, Elisabeth G.E. de Vries.

Quoting Paolo Tarantino’s post:
”How do we build a new generation of safer and more active ADCs (Antibody–Drug Conjugates) for treating cancer? By understanding how they actually work (and stop working). In this Cancer Discovery review, we retrace 40 years of ADC development, highlighting key lessons learned.
ADCs harbor 3 key elements: an antibody, a linker, a payload. Since the 1980s, ADCs mostly harbored microtubule and alkylator payloads. More recently, topo1 inhibitors led to major strides in oncology. Yet, the field is now getting saturated. Payload differentiation needed.
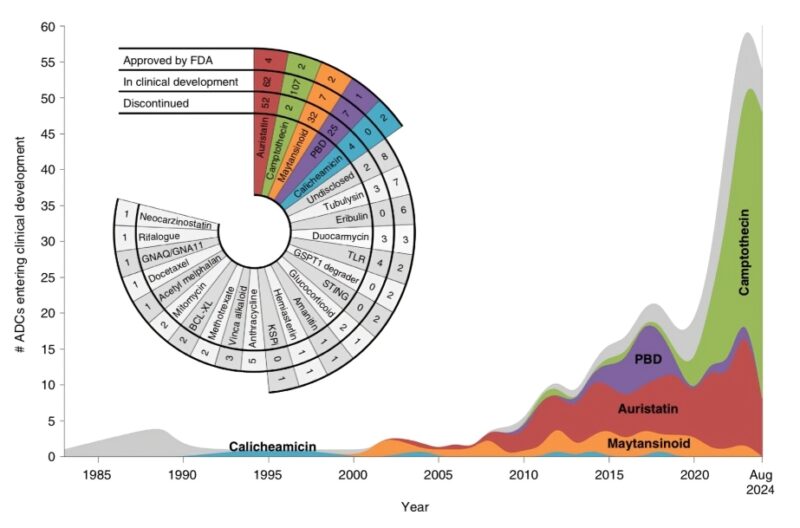
Notably, 40 years of development led to debunking of a common misconception. Indeed, most ADCs do not expand the therapeutic index of chemotherapy. Their tolerability is usually similar to chemotherapy. Yet, they can make chemotherapy much more effective, through an improved delivery.
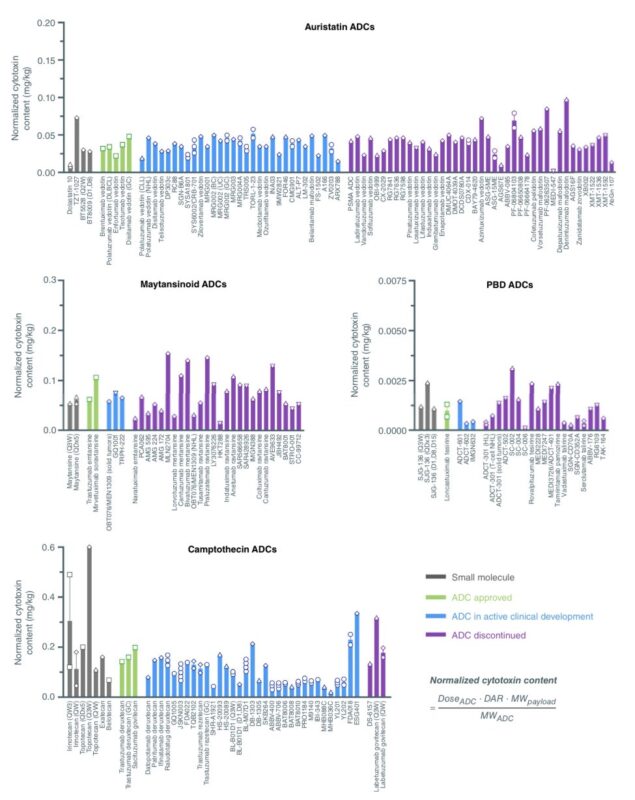
A second important misconception is that ADCs are stable. None of the currently approved ADCs is really stable. Which is a good thing. The continuous, dynamic deconjugation of payload from the antibody is emerging as a critical feature that makes ADCs so clinically active.
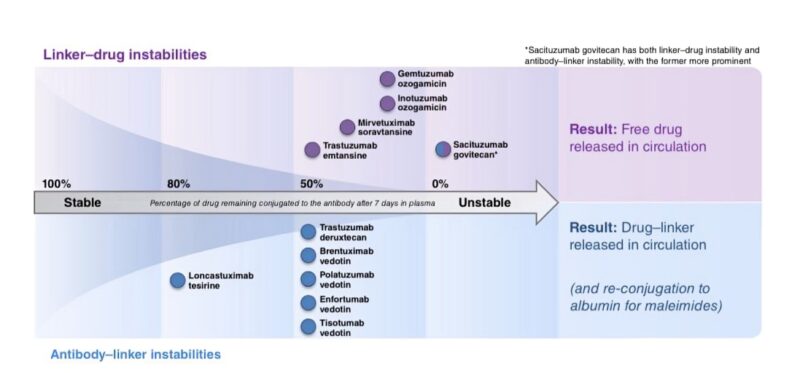
Indeed, it turns out that when you link a chemotherapy payload to an antibody, the chemotherapy acquires part of the pharmacokinetic features of the antibody. In short, you achieve an extended release of the chemotherapy, which is continuously released for days following each infusion.
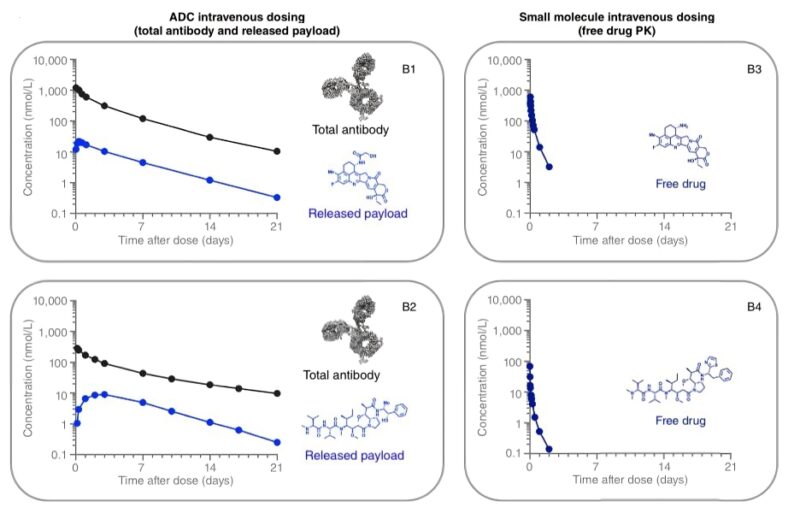
Upon this non-specific extended release, ADCs also enable a targeted delivery of payload. Notably, only 1% of the ADC reaches the tumor cell through target engagement. Yet, this can lead to major activity in cases where the target is highly overexpressed (eg. HER2+ tumors).
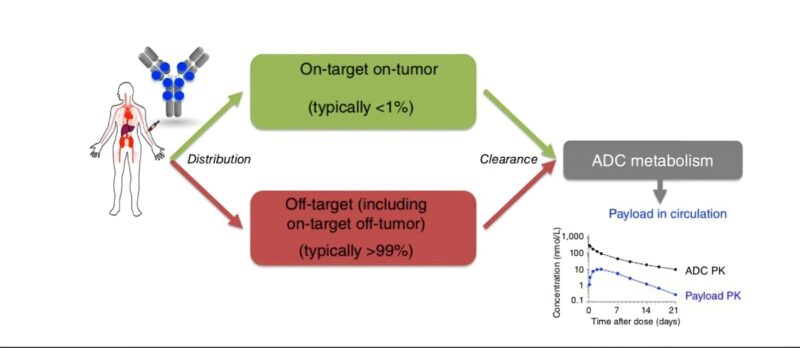
Yet, the above is just the tip of the iceberg. ADCs are complex. They also act via engagement of immune cells, blocking of oncogenic pathways, conjugation of payloads with albumin and multiple other mechanisms of action. Embracing complexity will be key to develop the new generation of ADCs.
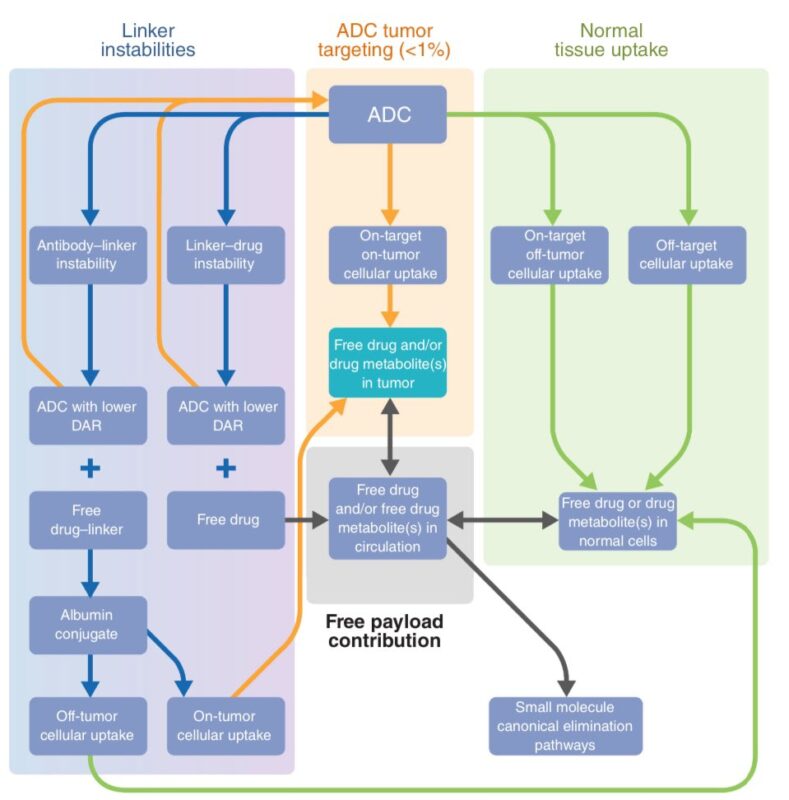
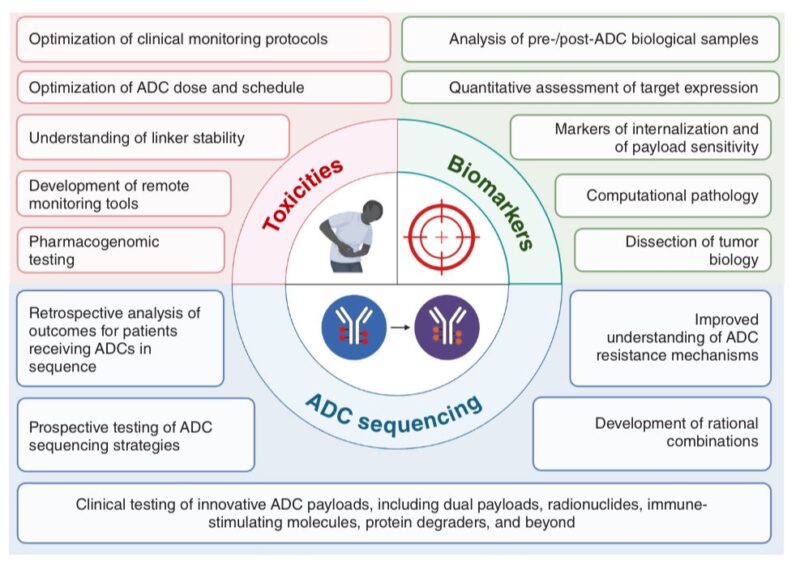
Their complexity translates in multiple challenges. Three major ones:
- Optimizing ADC sequencing,
- Minimizing toxicities,
- Developing better biomarkers.
Addressing these 3 key challenges will be critical to advance the field of ADCs over the next decades.
Can’t thank enough Raffaele Colombo for co-leading this review, Patricia M. LoRusso and Elisabeth de Vries for originally envisioning it, Jamie Rich and Sara Tolaney for the many insights during its development and the Cancer Discovery team for the outstanding editorial support at each step of its development.”
Quoting Raffaele Colombo’s post:
”Thrilled to see our ADC review online on Cancer Discovery!
…and perfectly timed for the start of ENASymp24.”
Sandra Misale, Assistant Professor at Sidney Kimmel Comprehensive Cancer Center, shared a post by Paolo Tarantino, on X, adding:
”Must read! Awesome work by Raffaele Colombo and Paolo Tarantino: all you need to know about antibody-drug conjugates.”
Matt Sagan, of FiBioMed, shared a post by Paolo Tarantino, on X, adding:
”Yes, 40 years of development for ADCs cancer treatment.
Antibody Drug Conjugates are 4 decades old, this is how much time it takes to get to the point where everybody talks about it! Same story with mRNA vaccines and many other platforms.
We investors or companies shareholders see only final few years when it lands into Phase 2 or 3 clinical trials or when its already on the market with WOW effect (f.e. Enhertu by AstraZeneca, Daiichi Sankyo US or Covid19 vaccines from BioNTech SE, Pfizer).
Beauty is to see this kind of novel approach much before, invest early, wait 10 years and cash on it – easy to say.”
Antonio Passaro shared a post by Paolo Tarantino, on X, adding:
”Congratulations to Paolo Tarantino for this important manuscript! A must-read for everyone.”
Paolo Tarantino, MD, is pursuing an advanced research fellowship at Dana-Farber Cancer Institute and Harvard Medical School, concurrently working towards a PhD in clinical research at the University of Milan. His research focuses on exploring the HER2 oncoprotein, investigating the emerging HER2-low subgroup of breast tumors, and developing novel antibody-drug conjugates targeting every subtype of breast cancer. With a publication record exceeding 50 papers on breast cancer, he is recognized as a leading expert in the field.
Raffaele Colombo, a leading figure in the pharmaceutical industry is the Associate Director of Medicinal Chemistry at Zymeworks Inc.. His leadership and scientific acumen drive the discovery and optimization of novel drug candidates and advancing the treatment landscape for various diseases.
Antonio Passaro, MD PhD, a medical oncologist at the European Institute of Oncology in Milan, specializes in lung cancer research and treatment. He focuses on developing new therapies, including immunotherapy, and identifying reliable biomarkers for non-small cell lung cancer (NSCLC). Dr. Antonio Passaro is an Associate Editor for Lung Cancer Journal by Elsevier and the Chair of the ESMO Press & Media Affairs Committee. He is also a faculty member for the Lung and Other Thoracic Tumours faculty group and the ESMO-MCBS Extended Working Group. He also holds editorial positions and key roles in societies like ESMO and AIOM.


