Patrick Forde, Professor at the Johns Hopkins University, shared a post on X by FDA Oncology, adding:
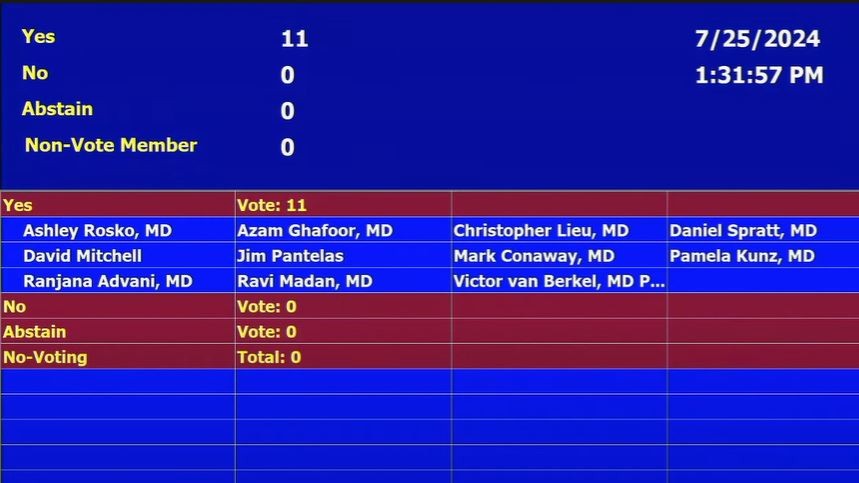
“Interesting ODAC (Oncologic Drugs Advisory Committee), future perioperative trial designs should consider contribution of phases but AEGEAN (neoadjuvant chemo-durva followed by adjuvant durva) looks like it will be approved. Would have been difficult not to do so post approval of KN671 with a very similar design.”
Quoting FDA Oncology’s post:
“Oncologic Drugs Advisory Committee votes 11 to 0 in favor of FDA requiring that new trial design proposals for perioperative regimens for resectable NSCLC include adequate within trial assessment of contribution of treatment phase.”
Link to video.
Sources: Patrick Forde/X and FDA Oncology/X