Svetlana Nikic, Founder of Precision Oncology Consulting, shared a post on LinkedIn:
“After a somewhat hectic start into ESMO – European Society for Medical Oncology, here’s what I’ve heard today at Day 1 of ESMO25:
- Patient quality of life (QoL) is increasingly decisive in HTA, but current data quality is insufficient.
- Only 2-3% of cancer RCTs actually meet the criteria for robust QoL scoring under ESMO-MCBS.
- Statistical rigor is the main failure point – missing data handling, no correction for multiple testing.
- Different regulators interpret QoL differently; companies must harmonize early.
- SISAQOL-IMI is becoming the de facto PRO standard across EMA, FDA, HTA, and patient groups.
- ESMO-MCBS is now widely used by HTA bodies – but needs to be contextualized with prognosis and disease setting.
- EU HTA Regulation (2025-2026) introduces Joint Assessments parallel to EMA approval – with public dossiers and mandatory patient involvement.
- Industry cannot apply directly to JSC – must go via patient or clinician orgs in the HTA stakeholder network.
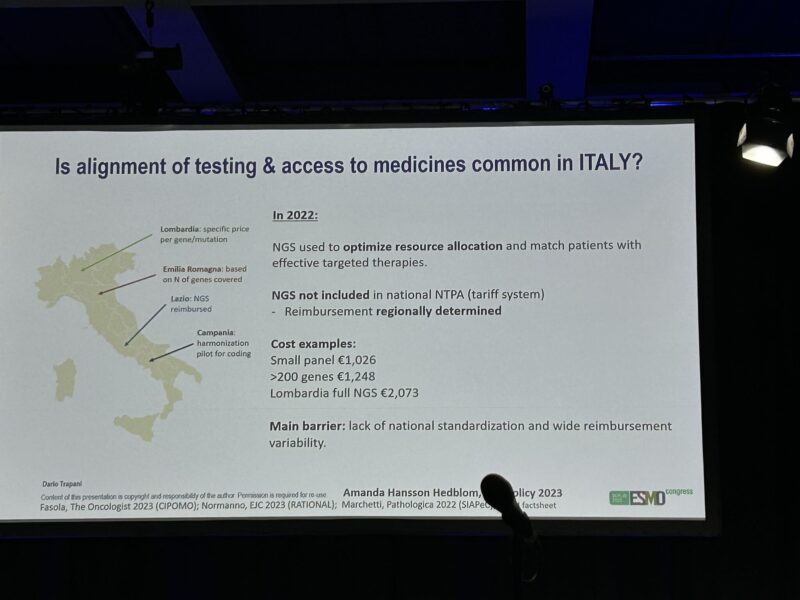
- Italy demonstrates a strong national adoption of ESCAT for biomarker funding – a central budget increase from 5M euros to 38M euros and full inclusion of ESCAT 1 alterations.
- Patient-based evidence is increasingly being positioned alongside clinical and economic data – not as a ‘soft add-on,’ but as a co-equal decision driver.
Please like and share if you find this useful.”
More posts featuring Svetlana Nikic on OncoDaily.


