Stephen V Liu, Director of Thoracic Oncology, Chief of the Division of Hematology and Oncology, and Associate Professor at Georgetown Lombardi Comprehensive Cancer Center, shared a post on X:
“Grateful to present an update on the phase II eNRGy trial at NACLC25: zenocutuzumab in patients with treatment-naive, NRG1-fusion positive NSCLC. Zenocutuzumab was FDA approved December 2024 in previously treated NSCLC and pancreatic cancer but did include some treatment naive.

Zenocutuzumab is a HER2/HER3 bispecific antibody that prevents NRG1 from binding to HER3 and triggering heterodimerization with HER2 and downstream signaling. eNRGy was a single arm phase II of zenocutuzumab 750mg IV q2w and included 20 patients with treatment naive NSCLC.

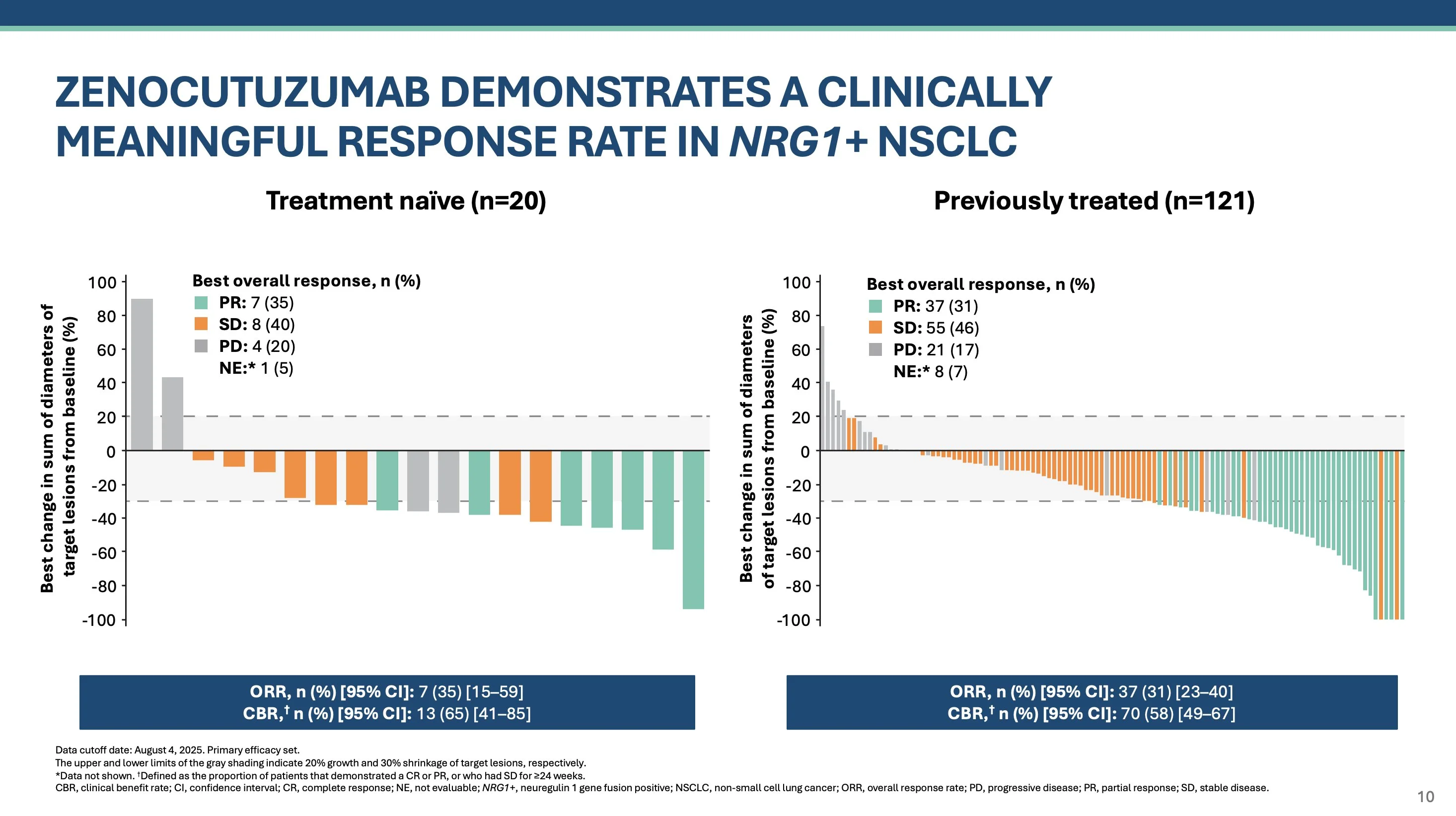
In treatment naive cohort, RR 35% with CBR 65% (here, clinical benefit defined as stable disease lasting at least 6 months). In my experience, NRG1+ NSCLC is difficult to measure due to frequent lymphangitic spread – I think CBR is a good reflection of benefit.
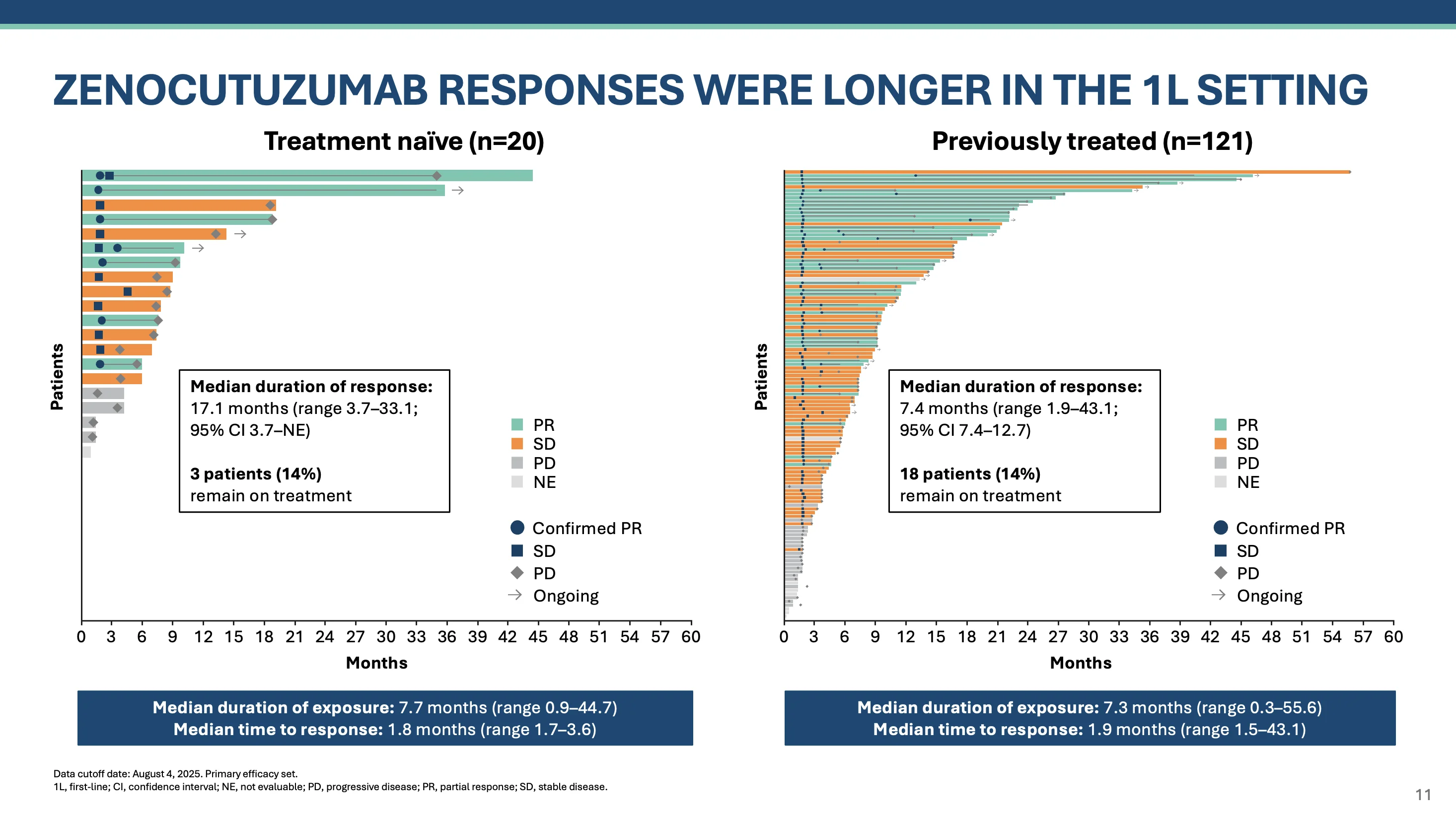
In eNRGy, zenocutuzumab responses were numerically longer in the 1L setting. Median duration of response was 17.1m for treatment naive NRG1+ NSCLC and 7.4m in previously treated setting. Median time to response 1.8-1.9 months.
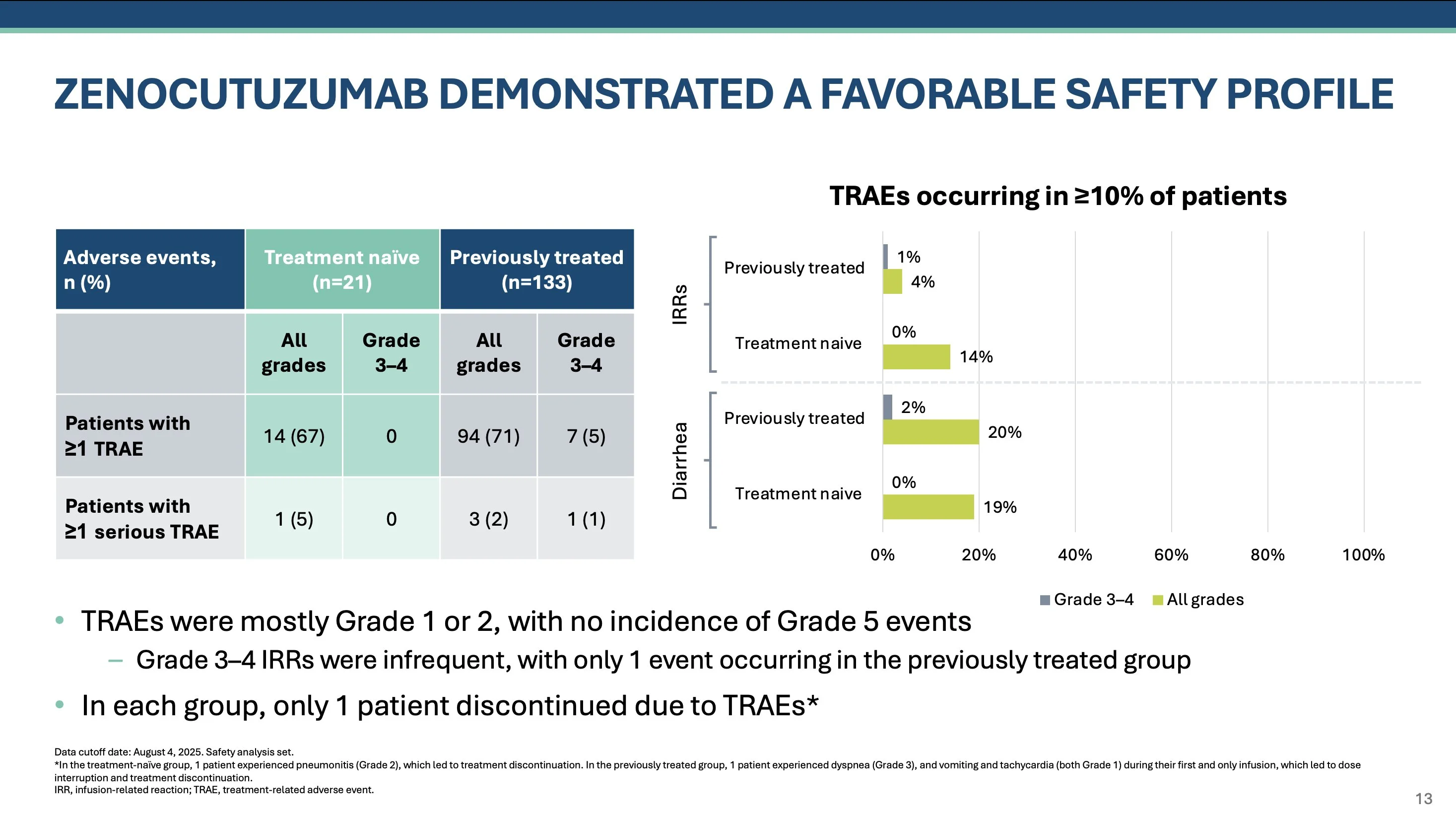
For treatment naive cohort, no G3-4 TRAEs and only 5% in previously treated cohort. TRAEs in ≥ 10% of pts were only infusion reactions (only 1 grade 3+) and diarrhea in ~20% (mostly G1-2). Only 1 patient in each cohort discontinued due to TRAE.”
More posts featuring Stephen V Liu.


