As ESMO25 approaches, Stephen Liu, MD – ESMO Ambassador, Director of Thoracic Oncology and Chief of Hematology and Oncology at Georgetown Lombardi – spotlights key thoracic oncology updates. From adjuvant alectinib in ALK+ NSCLC to next-generation KRAS G12C and EGFR exon 20 inhibitors, BRAF V600E follow-up, PD-L1 high immunotherapy trials, and MRD analyses, these data promise to shape the evolving lung cancer landscape.
ALINA Trial Landmark Data Preview
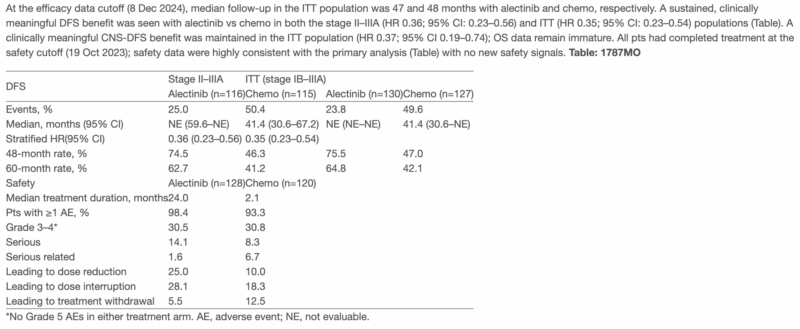
“ESMO25 will include update from ALINA: adjuvant 2y alectinib vs chemotherapy for resected ALK+ NSCLC (Abs 1787MO). With median follow up of 4y, DFS HR 0.35, CNS DFS HR 0.37, OS immature. Eager to see the curves, landmarks, especially given 2y course.”
Olomorasib Intracranial Efficacy at ESMO25
“ESMO25 Abstract 1846MO on Sunday Oct 19, Dr. Cassier Philippe will report intracranial efficacy of olomorasib (2nd gen KRAS G12C inhibitor). In pts with untreated brain metastases, intracranial RR 44.4%, icDCR 83.3%, icTTR 2m, icDOR and icPFS not reached.”

Zipalertinib CNS Efficacy at ESMO25
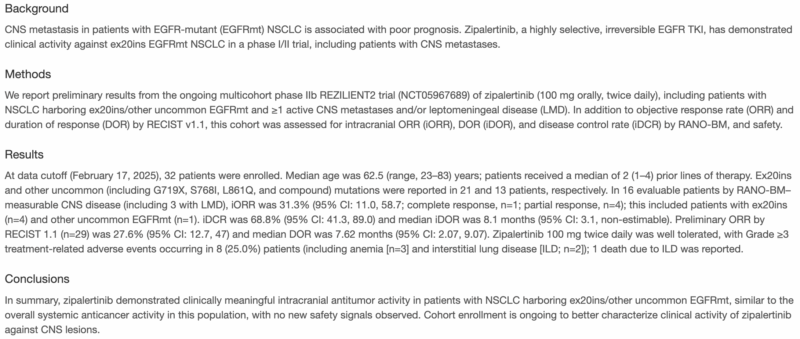
“ESMO25 Abstract 1847MO (Sunday Oct 19th), Dr. Helena Yu will show CNS efficacy of zipalertinib in EGFR exon 20 NSCLC from REZILIENT2 trial. Intracranial RR 31.3%, icDCR 68.8%, icDOR 8.1m. Important outcomes for this class.”
“ESMO25 Dr. Ying Liu will present abstract 1848MO, results from the phaes II FURMO-003 study of firmonertinib (aka furmonertinib) in pts with previously treated EGFR exon 20 NSCLC. ITT RR 44.3%, DCR 90%, DOR 8.3m, PFS 8.3m, OS 21.2m, diarrhea in 64.8%.”

“Some impressive followup at ESMO25 for BRAF V600E NSCLC. Dr. Melissa L. Johnson will update the phase II PHAROS study of encorafenib plus binimetinib with 52.3m follow up. As first-line, OS 47.6m, 4y OS rate 49%. For previously treated, OS 22.7m, 4y OS rate 31%.”

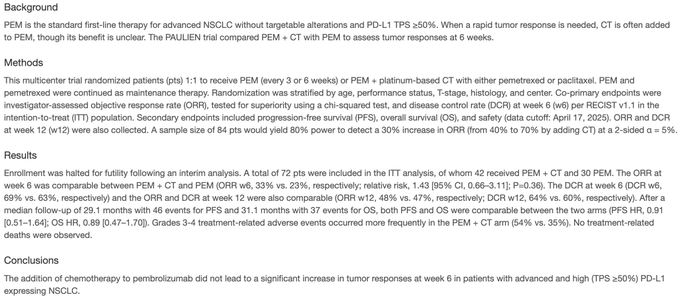
“ESMO25 Dr. Ilias Houda presents 1851MO, the PAULIEN trial: 1L pembro +/- chemo in PD-L1 high NSCLC. Stopped early for futility. RR at 6wks favored pembro/chemo (33% vs 23%) but no difference at 12wks (48% vs 47%). PFS HR 0.91, OS HR 0.89. G3+ TRAE 54% vs 35%.”
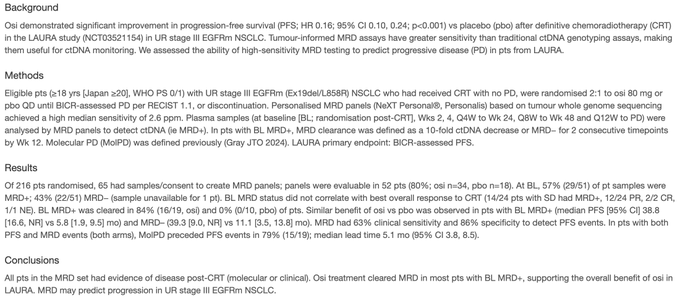
“ESMO25 Dr. Edurne Arriola Aperribay will present 1817MO on MRD in the LAURA trial (consolidation osimertinib after CRT for stage III EGFR NSCLC). At baseline, 57% were MRD+, 84% cleared, MRD with no impact on PFS. Molecular PD preceded radiographic PD by 5.1m.”
More posts from ESMO25.


