Sergio Cifuentes Canaval, Medical Oncologist at Las Américas Auna Clinic, shared a post on LinkedIn:
“ALASCA Phase 3 Trial: Low-Dose Aspirin in PI3K-Altered Localized Colorectal Cancer
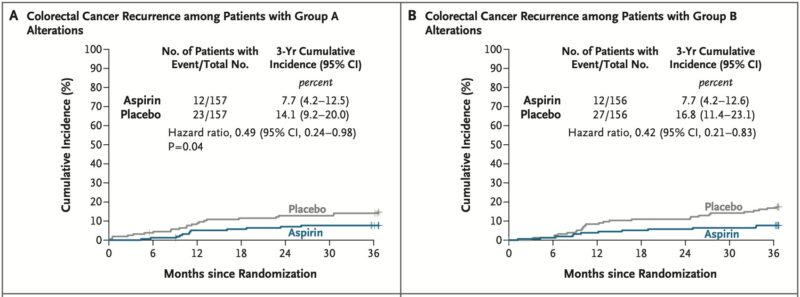
The ALASCA trial provides important new evidence on the potential role of aspirin in the adjuvant treatment of colorectal cancer with PI3K pathway alterations (PIK3CA, PIK3R1, PTEN).
Study design:
Patients (stage II–III colon or stage I–III rectal cancer) underwent radical resection.
Randomized within 12 weeks to receive aspirin 160 mg daily vs placebo for 3 years.
Group A: hotspot PIK3CA mutations (exons 9/20).
Group B: other PI3K/PTEN variants.
Key results:
3-year recurrence rates:
- Group A: 7.7% (aspirin) vs 14.1% (placebo); HR 0.49, p=0.04
- Group B: 7.7% (aspirin) vs 16.8% (placebo); HR 0.42
3-year disease-free survival:
- Group A: 88.5% vs 81.4% (HR 0.61; not significant)
- Group B: 89.1% vs 78.7% (HR 0.51; significant)
Conclusion:
Low-dose aspirin significantly reduced recurrence risk in patients with PI3K-altered localized CRC after surgery, with the strongest effect seen in Group B (non-hotspot PI3K/PTEN variants).
This trial reinforces the idea that an inexpensive and widely available agent could provide meaningful, molecularly targeted benefit in colorectal cancer.
A key question remains for our region: would a daily dose of 160 mg be tolerated in Latin American patients, where expanded use of lower doses was not considered in the trial?”
More posts from Sergio Cifuentes Canaval.


