Artak Heboyan, PhD, Associate Professor, Program Director of Dentistry at Yerevan State Medical University after Mkhitar Heratsi, Editor-in-Chief of The Journal of Basic and Clinical Dentistry (JBCD) Shared a New Invention in Oral Squamous Cell Carcinoma.
A new biomedical diagnostic invention has been officially registered (Design number: 6488066; Grant date: 02 December 2025).
Title: Cytotoxicity of Nanocarrier-Based Drug Delivery in Oral Cancer Therapy: A Systematic Review and Meta-Analysis
Authors: Mohammad A. Saghiri, Ravinder S. Saini, Artak Heboyan

The design features a nanoparticle-based salivary biosensor developed for early, non-invasive detection of Oral Squamous Cell Carcinoma (OSCC). This technology utilizes biomarker recognition, nanoparticle-driven signal amplification, and real-time analysis to support rapid and accessible cancer screening. The invention represents a significant advancement in next-generation biosensing and oral cancer diagnostics.
OSCC accounts for over 90% of all oral cancers and is predominantly diagnosed at late stages, leading to high morbidity and mortality. Current diagnostic methods, including biopsy, histopathology, imaging, and cytology, are invasive, expensive, and unsuitable for population-level screening. Saliva has emerged as a promising diagnostic fluid, containing numerous OSCC biomarkers, including CD44, IL-8, TNF-α, cytokeratin fragments, microRNAs, and extracellular vesicles. However, existing salivary biosensors suffer from low sensitivity, limited stability, interference from complex saliva matrices, and sometimes require bulky laboratory equipment.
There is an urgent need for a portable, ultra-sensitive, low-cost biosensor capable of detecting OSCC at early stages using non-invasive saliva samples. Nanomaterials provide excellent surface area, conductivity, and tunability, enabling highly sensitive biosensing applications. The present invention addresses these challenges by integrating functionalized nanoparticles, biospecific ligands, and electrochemical/optical transduction into a compact diagnostic platform.
The objectives of the invention are (1) to develop a rapid, portable biosensor for early OSCC detection using saliva, (2) to functionalize nanoparticles for selective recognition of OSCC biomarkers, (3) to provide a non-invasive, point-of-care diagnostic platform, (4) to enhance sensitivity via nanoparticle-based signal amplification, (5) to minimize sample volume, detection time, and operational complexity.
The invention introduces a nanoparticle-based salivary biosensor for detecting OSCC biomarkers with high accuracy and minimal sample preparation (Figure 1). The biosensor comprises:
- Nanoparticles (gold, magnetic, or graphene quantum dots) functionalized with OSCC-specific ligands.
- Biorecognition elements such as monoclonal antibodies, aptamers, peptides, or molecularly imprinted polymers (MIPs).
- Signal transduction module based on electrochemical impedance spectroscopy (EIS), fluorescence quenching, SERS, or colorimetric reaction.
- Microfluidic saliva processing chamber for filtration, concentration, and flow regulation.
Portable detection unit integrated with a smartphone interface or embedded microcontroller.
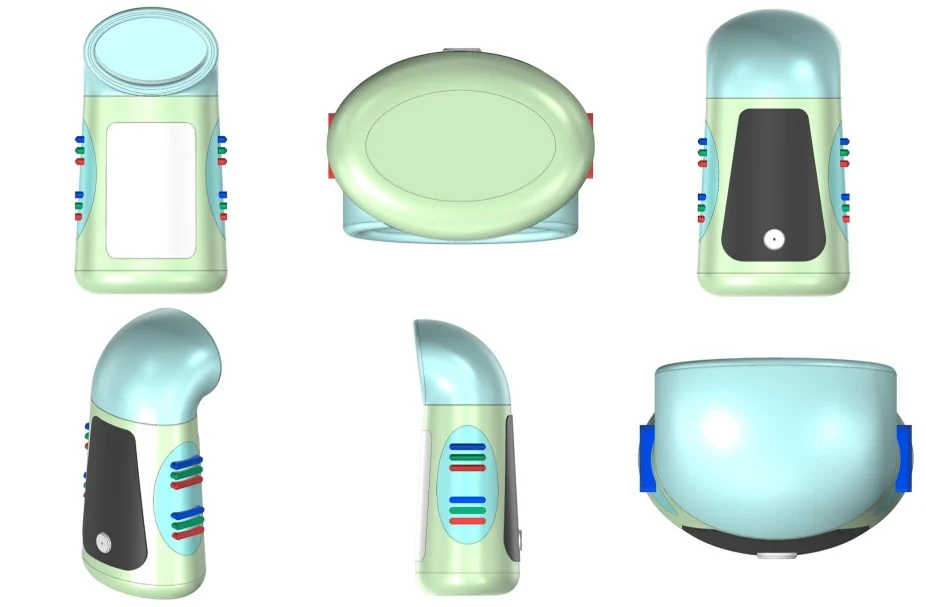 Figure 1. Invented salivary biosensor for detecting OSCC biomarkers.
Figure 1. Invented salivary biosensor for detecting OSCC biomarkers.
The biosensor detects specific biomarkers within minutes and provides quantitative results with enhanced sensitivity enabled by nanoparticle-mediated signal amplification.
The invention demonstrates several notable advantages, including non-invasive, saliva-based detection and exceptionally high sensitivity within the pg/mL to fg/mL range. Specificity is strengthened through the nanoparticle–ligand interface, enabling rapid point-of-care diagnosis. The method utilizes low-cost consumables and is suitable for mass screening, facilitating early detection of OSCC prior to the appearance of visible lesions.
The invention has broad potential applications, including use in hospital and clinical diagnostics, rural screening programs, and dental and ENT clinics. It may support oncology early-detection camps and facilitate personalized cancer risk monitoring. Additionally, the platform can be integrated into telemedicine systems to expand accessibility and continuity of care.
The invention is defined through a series of claims that outline its core components and diagnostic capabilities. It features a nanoparticle-based salivary biosensor comprising functionalized nanoparticles, biorecognition ligands, a saliva-processing microfluidic module, and a signal transduction unit for rapid detection of Oral Squamous Cell Carcinoma biomarkers.
The nanoparticles may include gold nanoparticles, magnetic nanoparticles, graphene quantum dots, silver nanoparticles, or silica nanoparticles, while the biorecognition ligands encompass antibodies, aptamers, peptides, or molecularly imprinted polymers specific to biomarkers such as CD44, IL-8, TNF-α, miRNA-21, and cytokeratin fragments. The biosensor operates using electrochemical, optical, Raman, or colorimetric signal transduction mechanisms and incorporates a microfluidic module with pre-filtration, controlled flow channels, an analyte concentration chamber, and an integrated sensing chamber.
A portable, smartphone-integrated reader unit is configured to process sensor output and quantify biomarker levels. Finally, the invention includes a diagnostic method involving saliva collection, microfluidic processing, nanoparticle-ligand interaction, signal detection, and biomarker quantification for early OSCC detection.
You Can Also Read: Common Side Effects of Oral Chemotherapy and How to Manage Them
Photo: Depositphotos