Sabine Brookman-May, Professor of Urology at Universität München, Senior Vice President and Therapeutic Area Head of Urologic Oncology at Aura Biosciences, shared a post on X:
“Great to have the chance to present efficacy and immune response/biomarker data from the first cohorts of our Phase 1 study with Belsar/AU-011 in NMIBC at SUO2025:
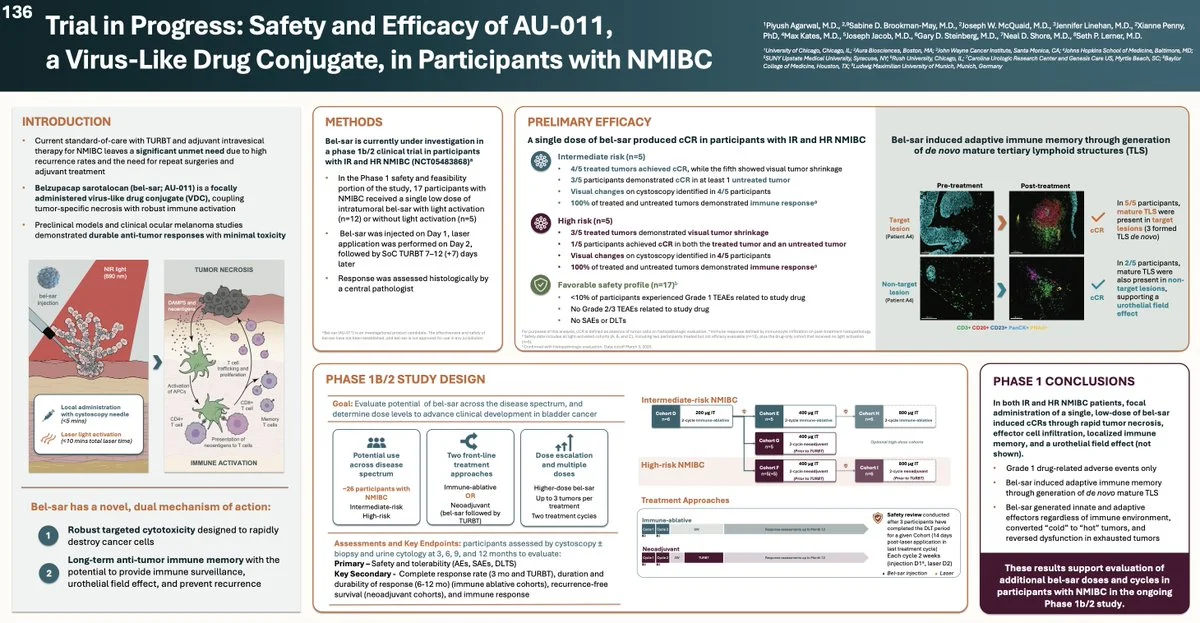
Bel-sar showed early CRs in both IR and HR NMIBC after one low intratumoral dose, with shrinkage in untreated lesions, pointing to an immune field effect.
Dual MoA: rapid targeted tumor necrosis + adaptive Immune Activation, including de novo TLS formation and conversion of cold into hot tumors.
Safety: only Grade 1 related AEs, no SAEs/DLTs.
A Phase 1b/2 dose-escalation study is now testing higher doses and 2-cycle regimens across IR/HR NMIBC to define the front-line potential of immune-ablative bel-sar as a potential neo-adjuvant treatment in Bladder Cancer.”
More posts featuring Sabine Brookman-May on OncoDaily.


