Roberto Iacovelli, Medical Oncologist at Gemelli Polyclinic Foundation IRCCS, and Associate Professor of Medical Oncology at Catholic University, Comprehensive Cancer Center of Rome, Italy, shared a post on X:
“Thrilled to share results from the TACITO trial, just out in Nature Medicine.!
FMT vs pbo in mRCC patients receiving pembrolizumab + axitinib.
We evaluated Fecal Microbiota Transplantation (FMT) vs Placebo in improving outcomes of 1st line pembrolizumab+axitinib in advanced renal cell carcinoma.
45 patients received FMT from a donor or Pbo within 2 months from the start of pembro/axi. FMT was given first by colonoscopy and then by frozen capsules after 3 and 6 months. The donor was a complete responder to immune checkpoint inhibitors (ICI).
While the primary endpoint (12-month PFS), was not met (70% vs 41% for d-FMT vs p-FMT, p=0.053), the median PFS was significantly longer with d-FMT (24.0 months in the d-FMT arm vs 9.0 months in the p-FMT arm, p=0.035).
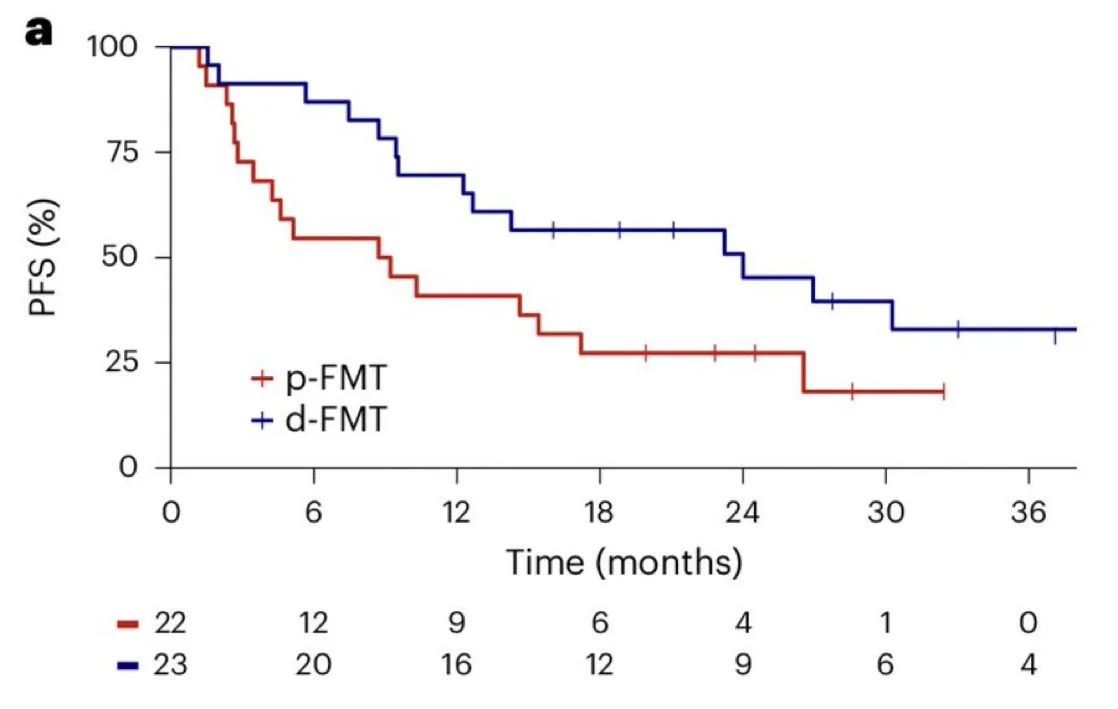
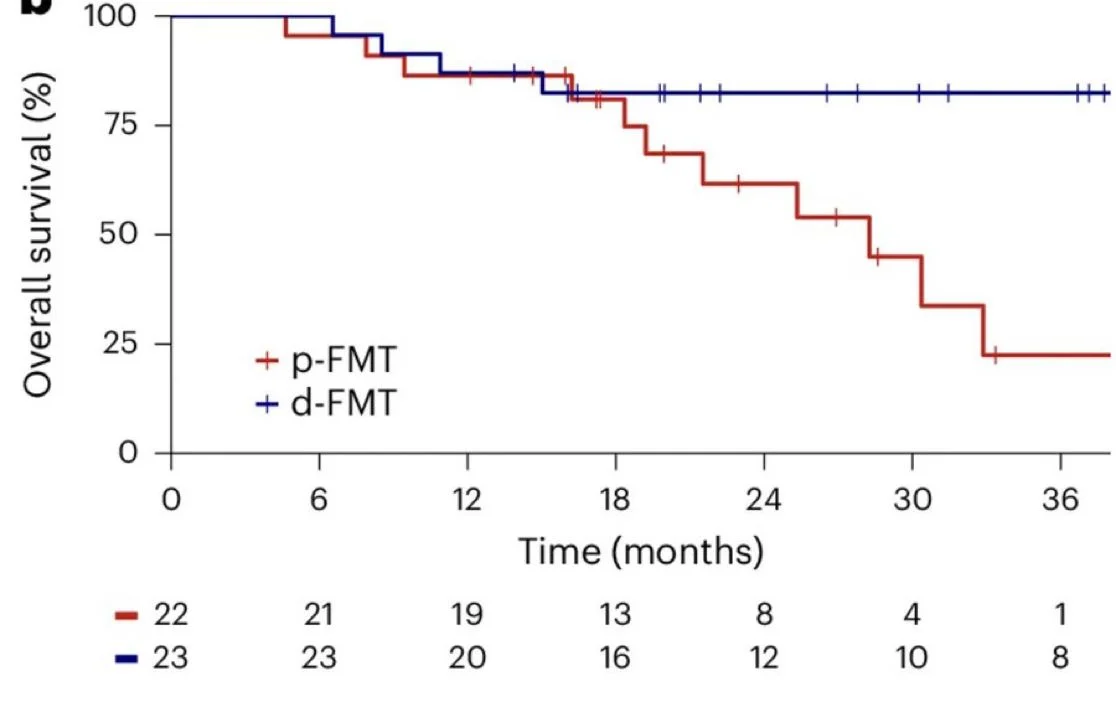
Median OS was longer in the d-FMT arm (41.0 months) vs the p-FMT arm (28.3 months) although not significantly (P = 0.167). ORR was 52% in the d-FMT arm and 32% in the p-FMT arm.
Interestingly, we found a greater benefit in patients with intermediate and poor prognosis: The 12-month PFS was 63% in the d-FMT arm and 27% in the p-FMT arm (P = 0.045). The mPFS was 18.8 months in the d-FMT arm and 5.1 months in the p-FMT arm (P = 0.033).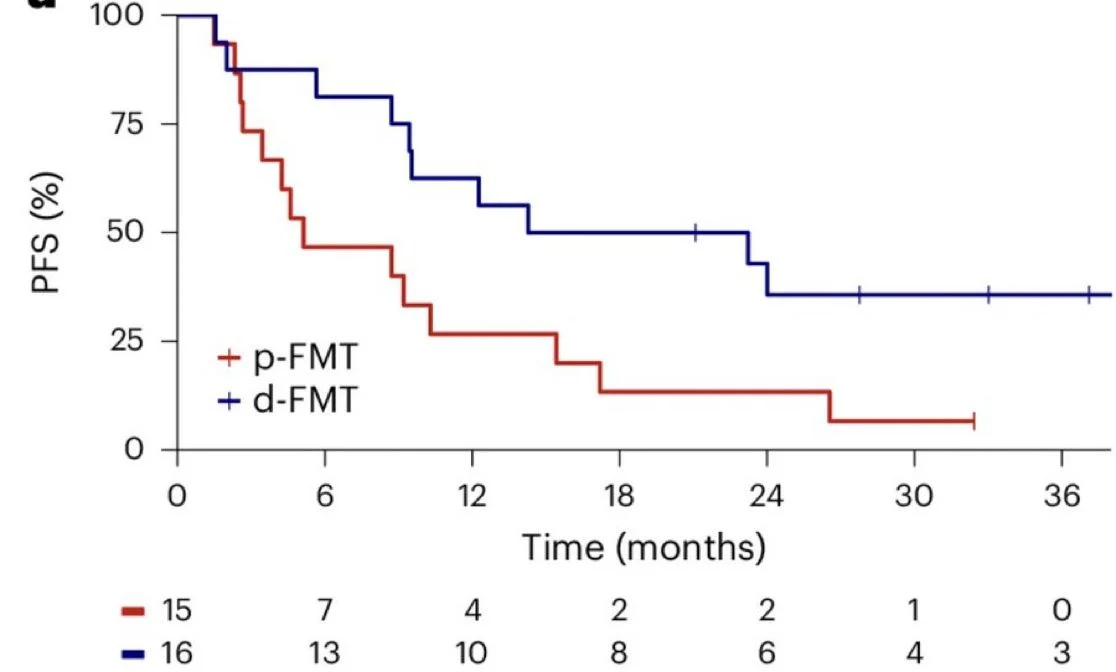
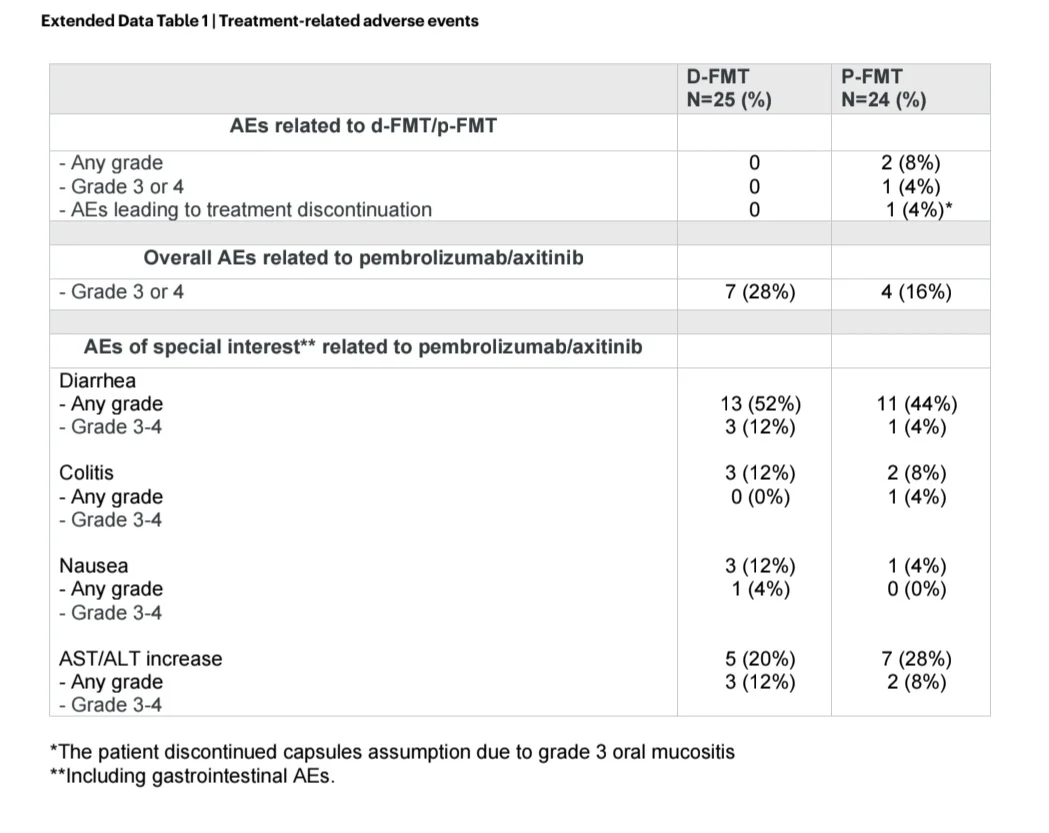
Notably, FMT was a safe procedure, as adverse events strictly related to the experimental procedures occurred rarely and only in the p-FMT group.
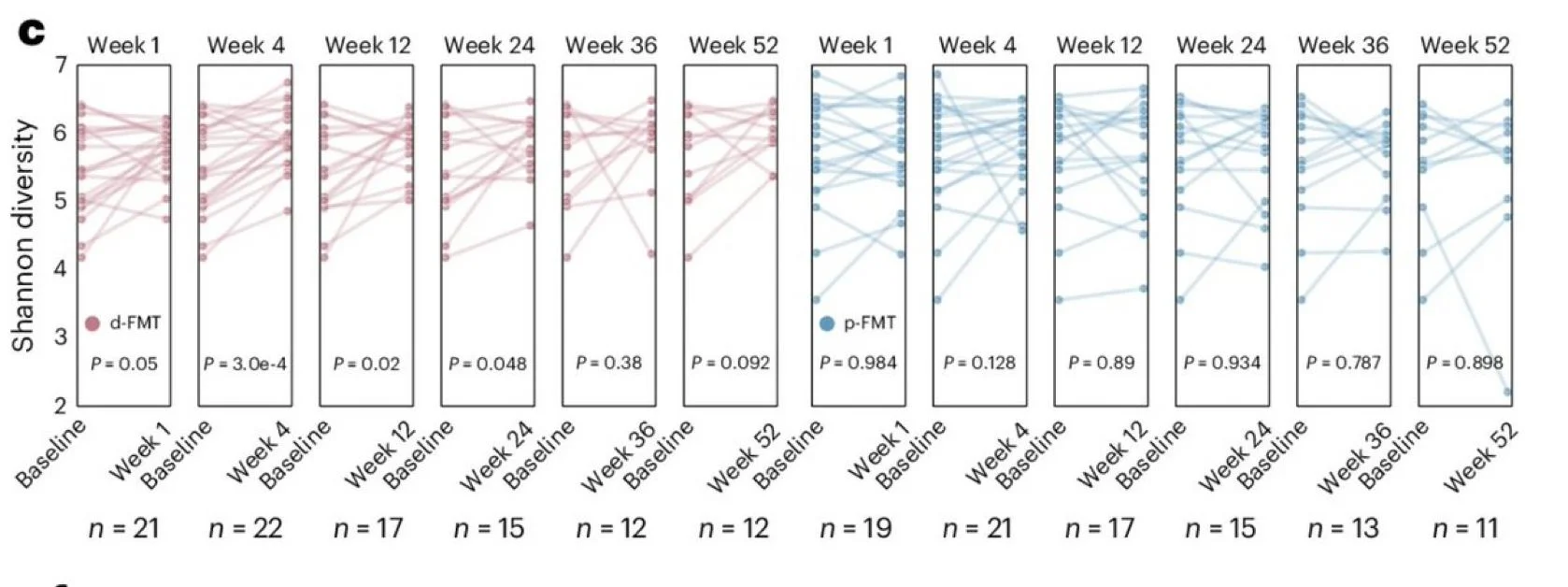
Microbiome analysis revealed that FMT was technically successful, as it confirmed donor strain engraftment and increased α-diversity and larger dissimilarity compared to baseline in the d-FMT group.
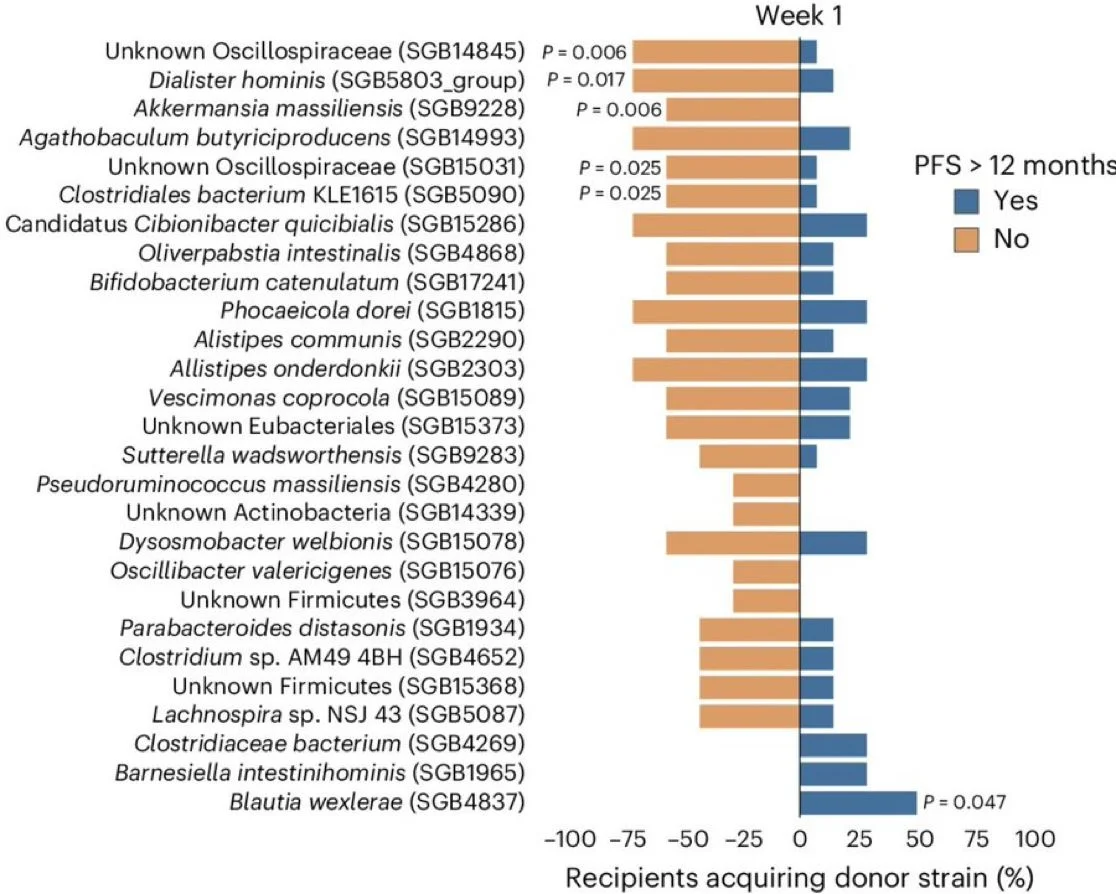
Interestingly, early acquisition of some species, e.g. Blautia wexlerae, was associated with achievement of the primary outcome.
Finally, also the loss of specific taxa, e.g. E. coli (SGB10068) was associated with 12-month PFS, suggesting that the displacement of some strains might contribute to clinical outcomes.
I really want to thank patients a their families, donors who showed a new way for lucky patients to helps their “colleagues” and the great Gastroenterology group at our Institution. This project (GR–2018–12365734) has been founded by Ministry of Health.”
Title: Fecal microbiota transplantation plus pembrolizumab and axitinib in metastatic renal cell carcinoma: the randomized phase 2 TACITO trial
Authors: Serena Porcari, Chiara Ciccarese, Vitor Heidrich, Debora Rondinella, Gianluca Quaranta, Andrea Severino, Daniela Arduini, Sebastiano Buti, Giuseppe Fornarini, Francesca Primi, Luciano Stumbo, Diana Giannarelli, Giulia Claire Giudice, Alessandra Damassi, Julio Rodrigo Giron Berríos, Michal Punčochář, Thomas B. Barbazuk, Gianmarco Piccinno, Federica Pinto, Federica Armanini, Francesco Asnicar, Giovanni Schinzari, Lisa Derosa, Guido Kroemer, Maurizio Sanguinetti, Luca Masucci, Antonio Gasbarrini, Giampaolo Tortora, Giovanni Cammarota, Laurence Zitvogel, Nicola Segata, Roberto Iacovelli, Gianluca Ianiro
Read the Full Article on Nature Medicine

More posts featuring Roberto Iacovelli


