Rahul Banerjee, Assistant Professor at the Fred Hutchinson Cancer Center and at the University of Washington, shared a post on X:
“As goes the ODAC, so goes the FDA! Excellent to see official MRD guidance in myeloma (even if I insist it’s measurable, not minimal). Guidance includes:
1. Use ITT analyses (denominator = all pts) Not “95% of all patients whom we thought would be MRD neg were so.
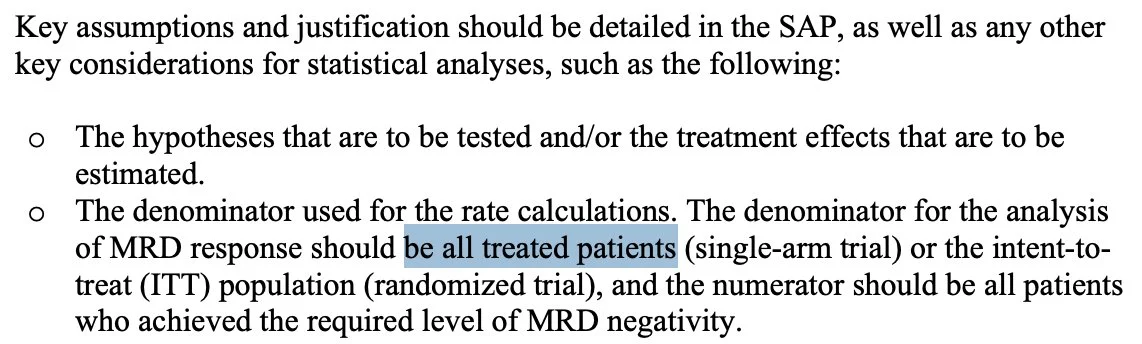
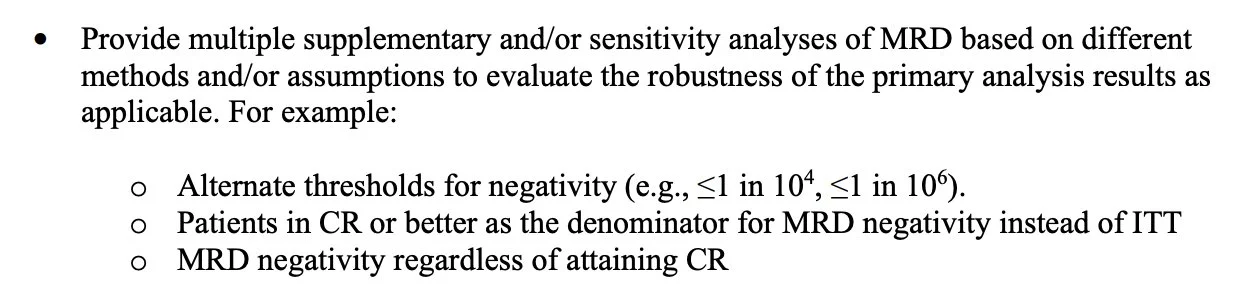
And 2: 1° endpoint should merge MRD-neg & CR into same endpoint (implicit rationale: enriches for true MRD-neg across marrow)
But “CR” will be a legacy term some day: so please report MRD-neg rates outside of CR somewhere in study!
Great FDA recs re: MRD in myeloma.
Only 13 pages long, so worth a quick read.”


