Pat Soon-Shiong, Chairman of Chan Soon-Shiong Family Foundation, Executive Chairman at ImmunityBio, and Executive Chairman of the Los Angeles Times, shared a post on X:
“Working to explain to the world how and why Bioshield works.
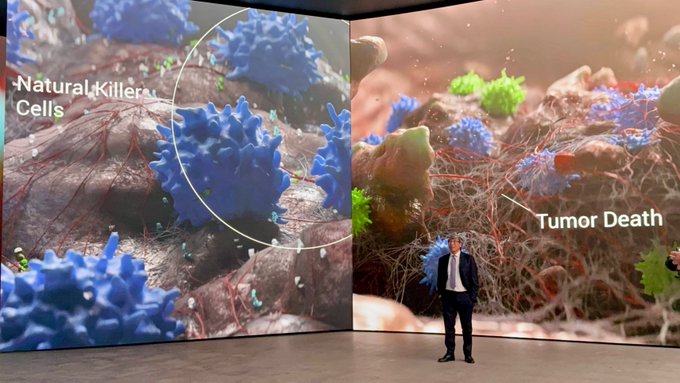
T cells and NK cells kill cancer, that’s irrefutable. Low NK cells and T cells means cancer grows, that’s irrefutable. So if there is a drug available to boost NK and T cells then there is better chance to kill the cancer cell of any tumor type – That’s the how and it is that simple.
- NK and T cells (lymphocytes) kill cancer… causation
- Anktiva proliferates, grows and activates NK and T cells in the body… causation (and in the FDA approved label)
- Adding Anktiva to current standards of care have shown prolonged survival in patients with cancer across multiple tumor types since it is treating the immune system… causation.
That is the HOW!
Anktiva the Bioshield is the first immunotherapy in history of medicine that is FDA approved with a mechanism of action to grow and supercharge NK and T cells in the body, generate memory T cells and restore the immune system without stimulating immunosuppressive T cells.
Now what’s next? We can measure low NK and T cells (lymphopenia) with a simple blood test (ALC). This is no different than the same test measuring low red blood cells (anemia) or low neutrophils (neutropenia). Low NK and T cells are called lymphopenia. Ask your doctor what is my ALC? (absolute lymphocyte count)
Today, FDA has approved EPOGEN to treat anemia regardless of cancer type and has approved NEUPOGEN to treat neutropenia regardless of cancer type. So, based on the mechanism of action of ANKTIVA and the irrefutable causation that NK cells and T cells kill cancer, ANKTIVA should be enabled to treat patients with low NK and T cells (lymphopenia) regardless of tumor type. Especially since ANKTIVA, by activating NK and T cells (reversing lymphopenia) is the only FDA APPROVED mechanism of action in the label to generate MEMORY T CELLS.
This supercharging of the immune system happens regardless of the cancer type and we have now shown in completed clinical trials across multiple tumor types including lung cancer (NSCLC), small-cell lung cancer (SCLC), urothelial carcinoma, head and neck cancer, melanoma, colorectal cancer, cervical cancer, renal cell carcinoma, gastric cancer, and hepatocellular carcinoma (manuscript in progress) that by increasing NK and T cells, we induce prolonged survival in patients who have failed all standards of care including current immunotherapy with checkpoint inhibitors.
This mechanism of action has already been approved showing long term complete responses, but approved only for an orphan state of a subset of bladder cancer.
So, if ANKTIVA could receive the same consideration as EPOGEN and NEUPOGEN as an immune supportive agent, many patients could benefit now without having to fly to LA. I hope we can change that quickly and change the lives of countless of desperate patients in which all standards of care have failed. Working hard to change the course of cancer everyday.
The paradigm shift: we are treating the immune system, not just the cancer. Cancer is the symptom; immune system collapse is the root cause.”
More from Pat Soon-Shiong on OncoDaily.


