Minhua Chu, Managing Partner at Transition Value Partner, shared a post on X:
“Using ADCs to deliver RAS inhibitors has emerged as a hotspot in drug development due to its ability to broaden the therapeutic window and minimize off-target toxicity.
According to patent information (WO2025/051241 A1), GenFleet Therapeutics is developing an EGFR/HER3 bispecific antibody-ADC carrying a pan-RAS inhibitor payload.
Now, at the recent AACR-NCI-EORTC 2025 conference, Adlai Nortye $ANL unveiled its Pan-RAS(ON)-conjugated ADC candidate AN4035.
Read more.
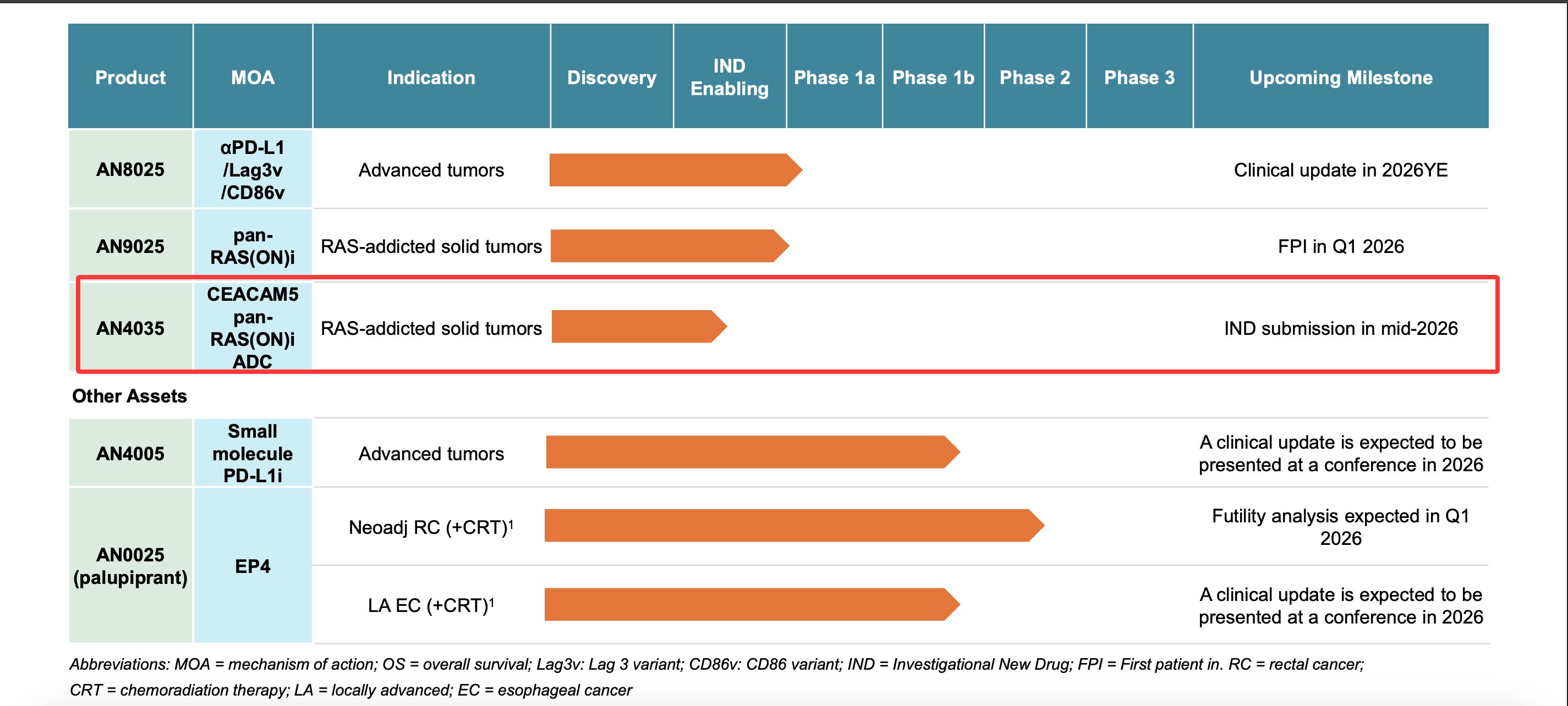
AN4035’s Payload
Exhibits activity comparable to RMC-6236
Mechanism: Binds CypA → forms ternary complex → inhibits RAS signaling Active against G12C, G12D, G12V mutations and wild-type KRAS, demonstrating potent cytotoxicity across multiple tumor cell lines.
AN4035 Design
Antibody: Anti-CEACAM5
Conjugation: Highly stable linkage with hydrophilic linker
DAR: 8
Serum stability: Free payload <0.06% after 14-day incubation
Intracellular Pharmacodynamics
Payload detected in cell lysates of CEACAM5-positive cells within 20 minutes
Ternary complex forms exclusively intracellularly
Binary KD (CypA): 14.9 nM Ternary KD (KRAS-G12D): 4.7 nM → ~3-fold enhancement, exhibiting classic molecular glue characteristics
Bystander Effect
Cytotoxic against 20 cancer cell lines regardless of KRAS mutation status
Stronger bystander killing compared to topoisomerase inhibitor payloads
Efficacy
HPAC and CL40 CDX models: – 3 mg/kg single dose → 78% tumor regression – 10 mg/kg → 85–89% regression
Across 26 NSCLC, CRC, and pancreatic cancer PDX models: Overall antitumor response rate of 73%
Preliminary PK/Tox
Mouse half-life: 8–9 days Cynomolgus monkey preliminary toxicology: No notable skin or GI toxicity; extremely low free payload exposure (AUC = 5.4 h·μg/mL)”
More posts featuring Minhua Chu on OncoDaily.


