Minhua Chu, Managing Partner at Transition Value Partner, shared a post on X:
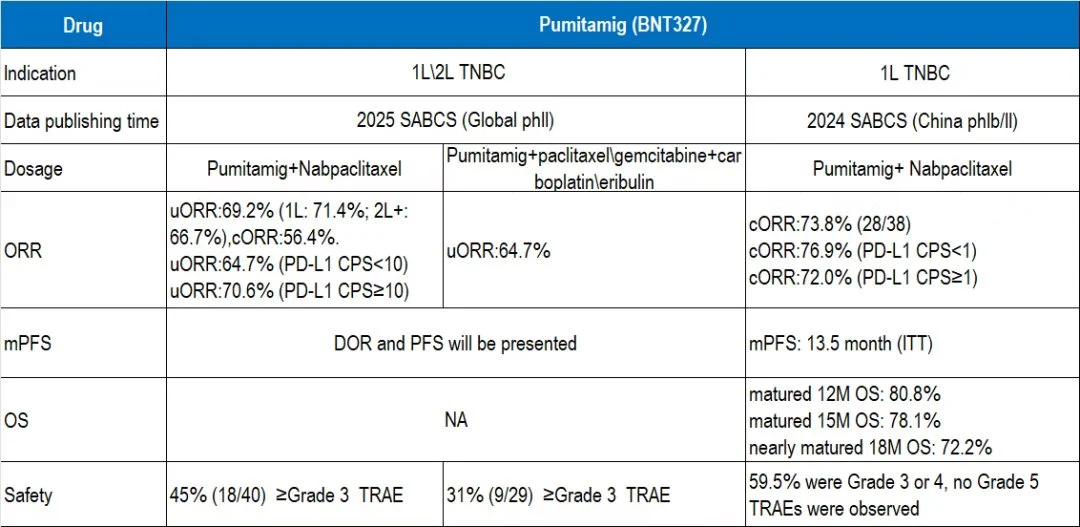
“BNTX will present first Phase 2 data of PD-L1/VEGF bispecific antibody pumitamig (BNT327) in TNBC.
In the study, dose exploration was expanded across 1L and 2L TNBC:
Cohort 1: 15 mg/kg or 20 mg/kg + nab-paclitaxel
Cohort 2 (multi-arm): fixed dose + paclitaxel, gemcitabine+carboplatin, or eribulin
Key efficacy results:
- Cohort 1 overall ORR: 69.4% (1L 71.4%, 2L 66.7%); confirmed ORR: 56.4%– Slightly lower than earlier Chinese 1L data but maintained strong responses even in PD-L1-low subgroups
- Cohort 2 overall ORR: 64.7%– Additional DoR and PFS data expected during the meeting
Safety highlights: Grade ≥3 treatment-related adverse events in both cohorts were lower than previously reported in Chinese early-phase studies, further confirming an improved tolerability profile.”
More posts featuring Minhua Chu.


