Mashukur Rahman, Clinical Oncology Resident, Physician, FCPS (Internal Medicine) (Final Part), BCS Health Cadre, Committed to Cancer Research, Patient Care, learning Oncology shared a post on LinkedIn:
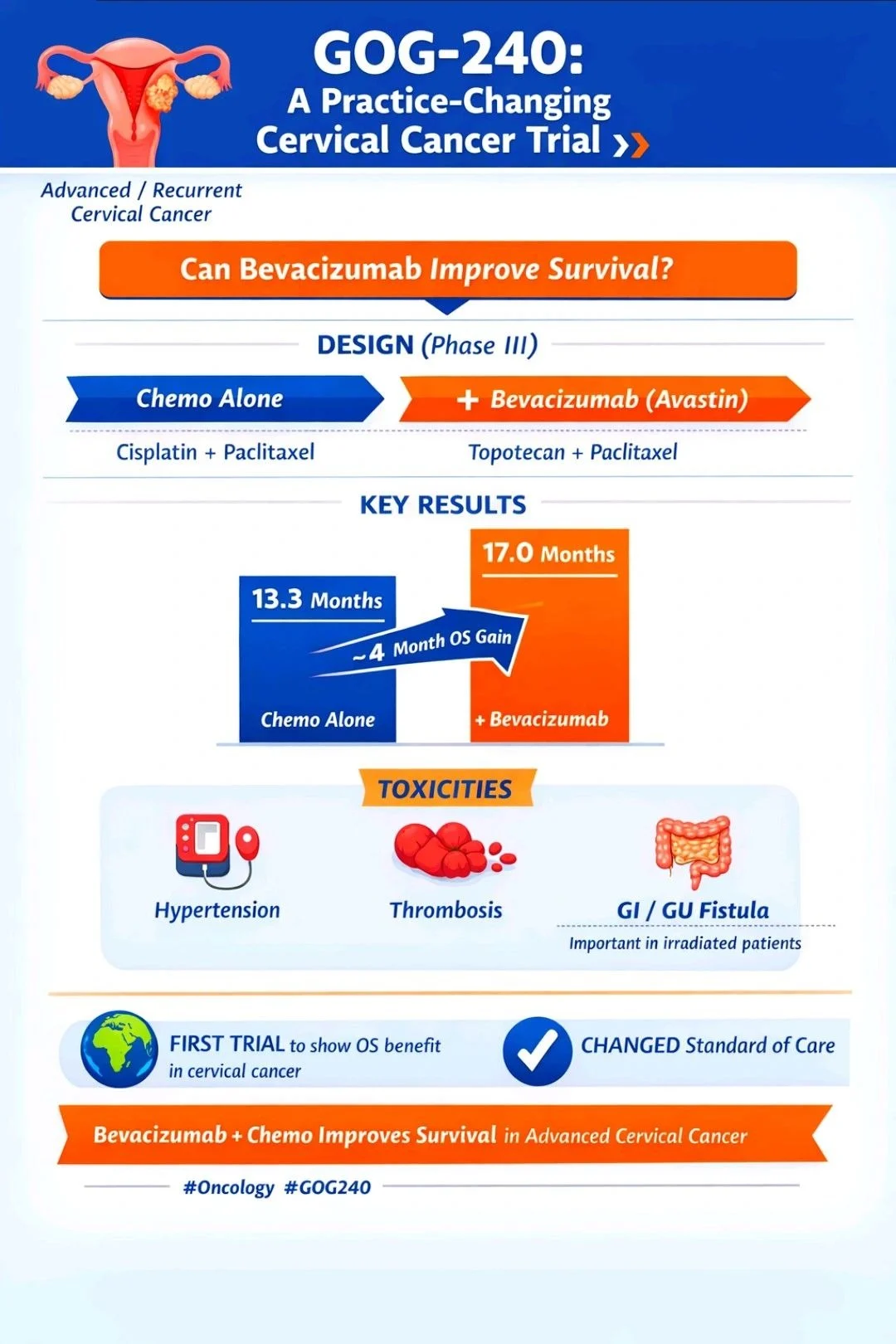
“Practice-Changing Trial in Cervical Cancer: GOG-240
For years, outcomes in advanced/recurrent cervical cancer were stagnant.
Then came GOG-240 — a true landmark.
What did GOG-240 ask?
Can adding Bevacizumab to chemotherapy improve survival?
Design (Phase III):
Chemotherapy ± Bevacizumab
(Cisplatin–Paclitaxel or Topotecan–Paclitaxel)
Key Result:
Overall Survival improved
- Chemo alone: 13.3 months
- Chemo + Bevacizumab: 17.0 months
~ 4-month OS gain
Toxicities to remember: Hypertension, thromboembolism, GI/GU fistula (especially in previously irradiated patients)
Why it matters:
- First trial to show OS benefit with targeted therapy in Ca cervix
- Changed global standard of care
One-line takeaway: GOG-240 = Bevacizumab + chemo improves survival in advanced cervical cancer.”
More posts about Cervical Cancer.


