Mashukur Rahman, Clinical Oncology Resident, Physician, FCPS (Internal Medicine) (Final Part), BCS Health Cadre, Committed to Cancer Research, Patient Care, learning Oncology shared a post on LinkedIn:
“Clinical Update: New Standards in Cervical Cancer Care
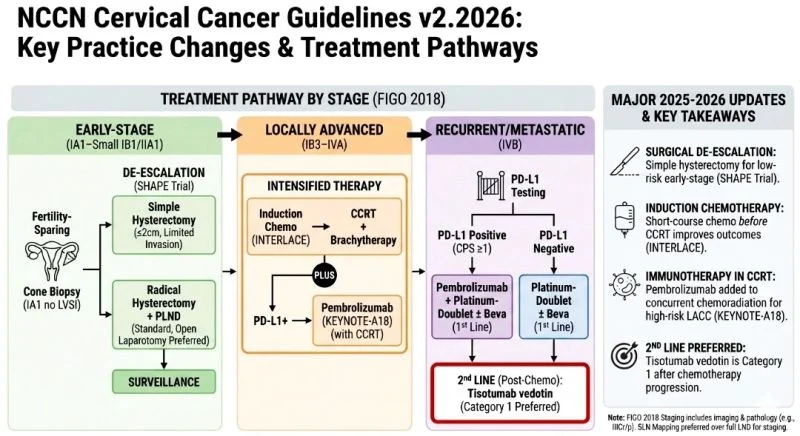
The 2026 NCCN updates focus on
three “P”s:
Precision in surgery,
Potency in chemoradiation, and
PD-L1 directed therapies.
1. Surgical De-escalation (The SHAPE Trial)
For “low-risk” early-stage disease (lesions ≤ 2 cm with limited invasion), simple hysterectomy is now a recognized alternative to radical hysterectomy.
2. Intensified Front-line Treatment (Locally Advanced)
We are moving beyond “standard” chemoradiation.
Two major additions for Stage IB3–IVA disease:
- Induction Chemotherapy: Short-course carboplatin/paclitaxel before CCRT (the INTERLACE regimen) is now a preferred strategy to improve survival.
- Immunotherapy: Adding Pembrolizumab to concurrent chemoradiation
3. Advanced & Recurrent Disease
- 1st Line: PD-L1 testing remains mandatory. Pembrolizumab + Platinum-doublet ± Bevacizumab is the gold standard for PD-L1 positive tumors.
- 2nd Line: Tisotumab vedotin-tftv has solidified its place as a Category 1 preferred recommendation for patients progressing after first-line therapy.
The integration of immunotherapy into earlier stages of treatment and the move toward less invasive surgery for early-stage patients are the defining shifts of this update.”
More posts featuring Mashukur Rahman on OncoDaily.


