Liang Cheng, Director of Anatomic Pathology and Director of Molecular Pathology at Lifespan Academic Medical Center, Vice Chair for Translational Research at the Warren Alpert Medical School of Brown University, and President at the International Society of Urological Pathology, shared a post on LinkedIn about a paper he co-authored with colleagues published in Histopathology:
“Honored to be part of this study, “Development and Preclinical Evaluation of a Novel FGFR3-Targeted Antibody–Drug Conjugate in Bladder Cancer,” published last month in Asian Journal of Pharmaceutical Sciences (Impact Factor: 11.9).
In this elegant work, Dr. Wang and colleagues report the design and synthesis of the first FGFR3-targeted ADC, LZU-WZLYFG001, developed specifically for the treatment of bladder cancer. This novel ADC demonstrated impressive tumor-targeting capability, efficient internalization with intracellular payload release, potent direct antitumor activity, and additional tumor-inhibitory effects through a bystander mechanism. Notably, it showed efficacy in tumors resistant to current clinical therapies while maintaining an excellent safety profile.
Together, these attributes not only fulfill the original design objectives but also position LZU-WZLYFG001 as a highly promising candidate for future clinical translation.”
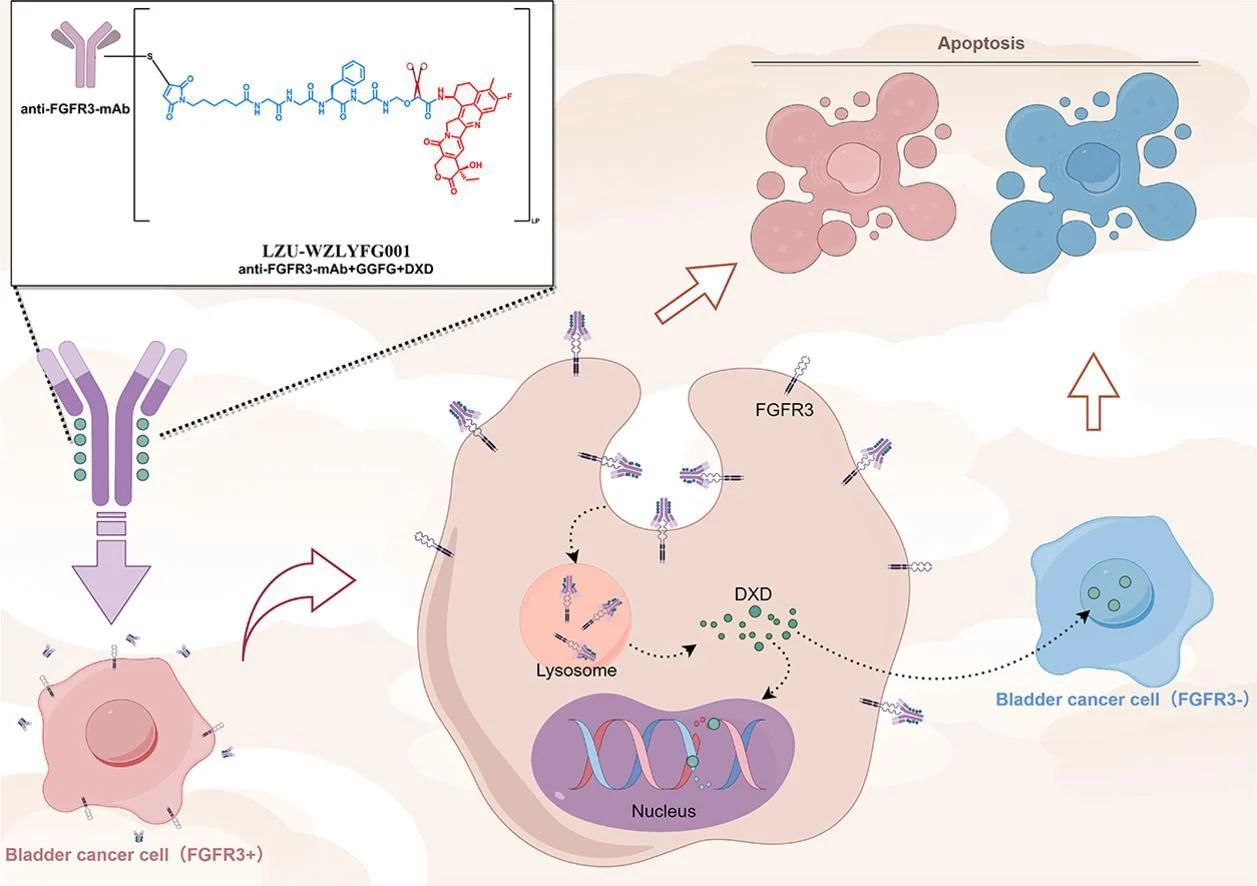
Title: Development and preclinical evaluation of a novel FGFR3-targeted antibody-drug conjugate in bladder cancer
Authors: Guangrui Fan, Xiongfei Luo, Kun Li, Ze Zhang, Chaohu Chen, Yibo Shi, Shu Cui, Yingru Wang, Dengtuo Wang, Zhijun Zhang, Zhilong Dong, Junqiang Tian, Liang Cheng, Juan Wang, Zhenxing Zhai, Yingqian Liu, Zhiping Wang

More posts featuring Liang Cheng on OncoDaily.


