Jia Jenny Liu, Translational Lead of Early Phase Drug Development at The Kinghorn Cancer Centre, shared a post on LinkedIn:
“In drug development, the biggest challenges are rarely the science alone. They sit at the interfaces.
Working across clinical oncology and translational research, I keep seeing the same gaps: strong research evidence that doesn’t translate into real-world access, novel therapies that are difficult to operationalise beyond sponsored trials, and regulatory approvals that outpace system readiness for patients.
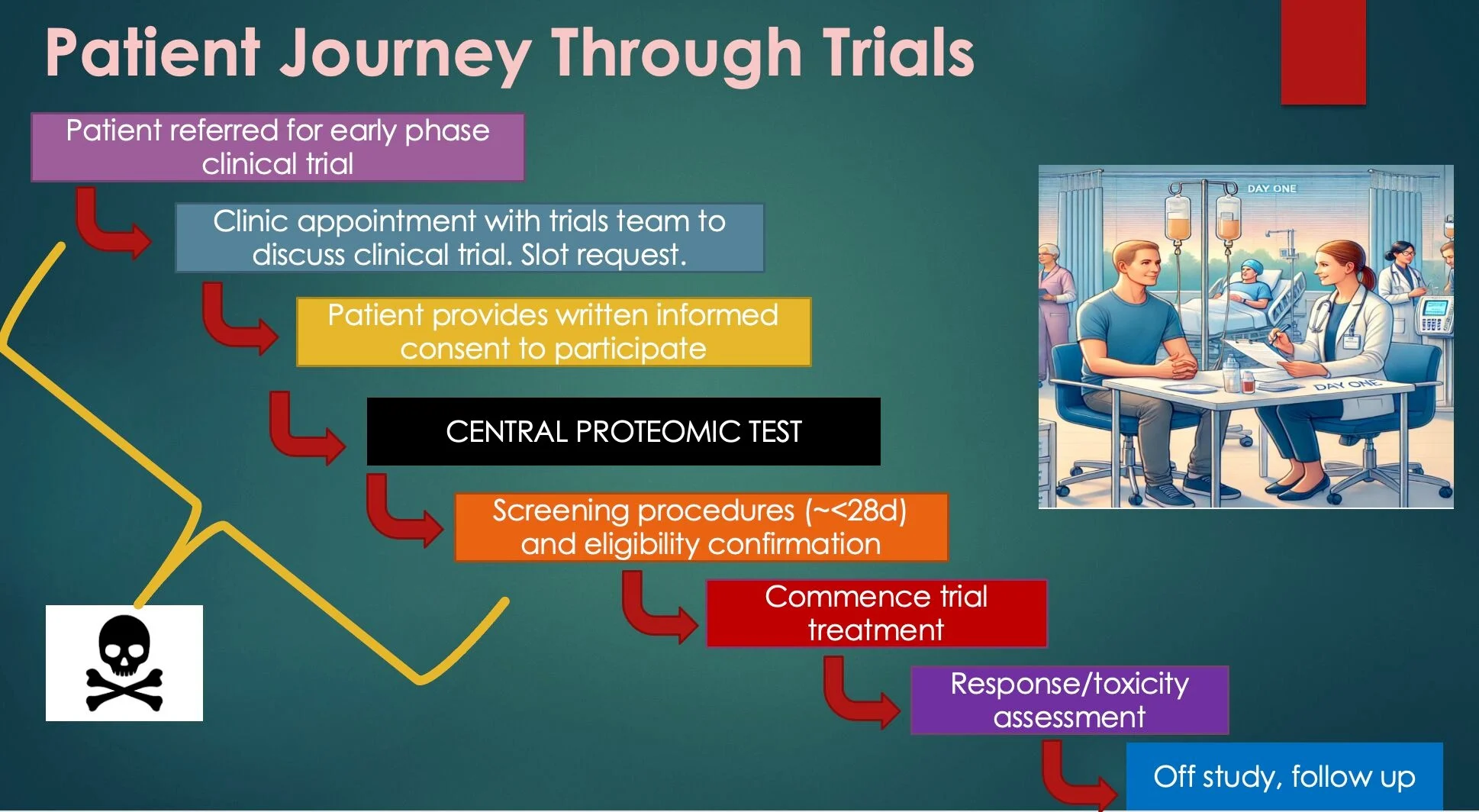
The images here reflect three recurring implementation challenges I’ve spoken about over the past year:
- tissue prescreening delays in early-phase trials that drive screen failures and lost opportunities;
- a growing technology cliff where biomarker sophistication outpaces feasible clinical decision-making;
- and the need for deliberate frameworks to bring tumour-agnostic therapies from signal to standard of care.
I’m excited to start 2026 joining the Editorial Board of JCO Oncology Practice for American Society of Clinical Oncology (ASCO). Editorial platforms play a powerful role in shaping how evidence is generated and translated, and I’m keen to support work that bridges research innovation with real-world access.
Always interested to hear how others are approaching these challenges in their own settings.”
More posts featuring Jia Jenny Liu on OncoDaily.


