Jia (Jenny) Liu, Translational Lead of Early Phase Drug Development at The Kinghorn Cancer Centre, shared a post on LinkedIn:
“One of the most insightful sessions for me at ESMO25
No its not another shiny new drug… which has groundbreaking efficacy but not access in the real world…
The educational session ‘Global perspectives, global promises: Regulators meet HCPs‘ really stood out for me. As a phase 1 oncologist I see so much inefficiency, death by paperwork, which is delaying trial conduct, increasing burnout in our trials team, and delaying access by increasing cost of drug development.
This session touched on:
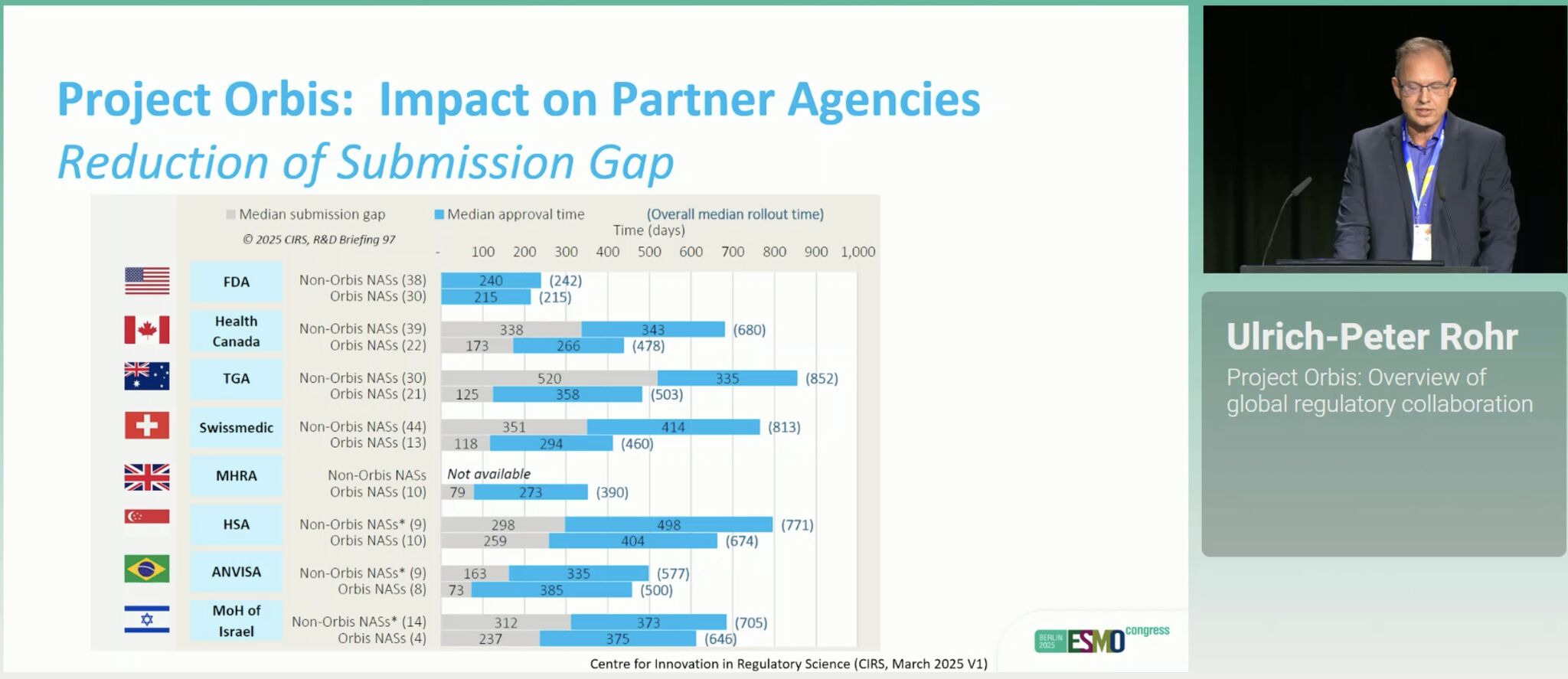
- The impact of regulatory harmonisation through efforts like Project Orbis to reduce the submission gap with >500 drugs reviewed by this collaboration since 2019.
- Pragmatic elements of trials need to be embraced; its a spectrum not a yes/no approach, and can improve patient centric trial conduct without compromising safety and efficacy
- The burden of research beaurocracy in research and impact at site/investigator level. Initiatives to simply delegation logs and data needed/not needed at different phases of trials will be removing the ‘pebble in the shoe’ that is causing us to waste resources and TIME.
Some key slides but if you have access to ESMO25 the discussion is very worth listening to.”
More posts from Jia (Jenny) Liu.


