Jean-Charles Soria, SVP and Oncology Therapeutic Area Head at Amgen, shared a post on X:
“2025 FDA approvals: oncology still leads – but with a shifting mechanistic profile
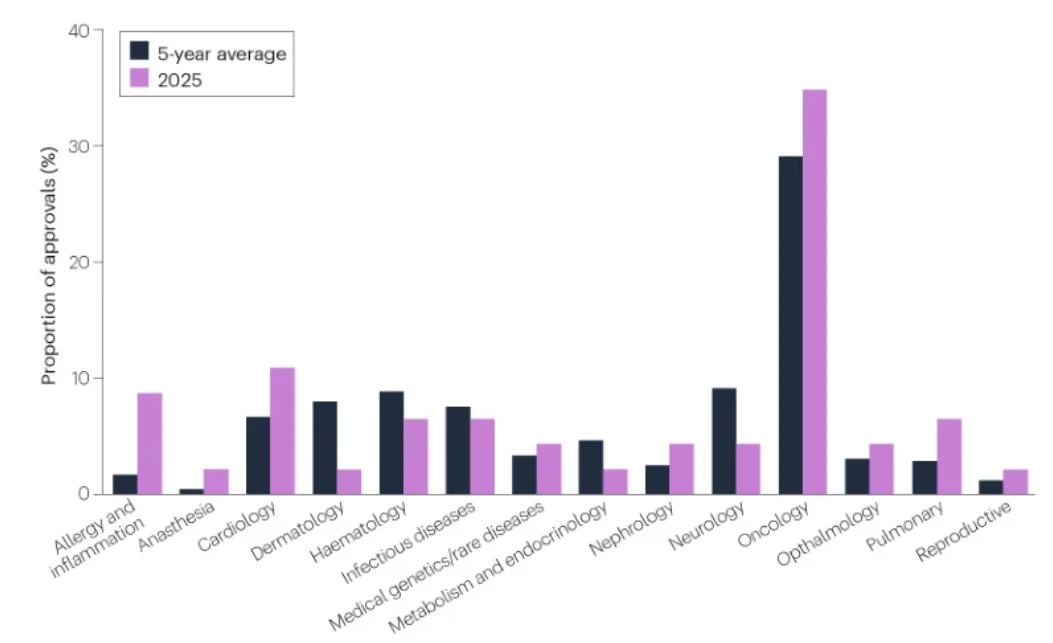
- Oncology dominates approvals: 16/46 (35%) new FDA drugs in 2025 were cancer therapies – well above any other therapeutic area, reinforcing oncology as the primary engine of regulatory innovation.
- MoA trend in oncology: approvals cluster around precision modalities – ADCs (TROP2, c-MET), bispecific T-cell engagers (BCMA×CD3), immune checkpoints (PD-1), and highly selective kinase inhibitors.
- Contrast with non-oncology TAs: cardiology, inflammatory and metabolic drugs largely target physiologic pathway modulation (BTK, DPP1, PCSK9, Naᵥ1.8), with fewer cytotoxic or immune-redirecting mechanisms.
- Biology-driven cancer approvals: multiple first-in-class or mutation-defined indications (H3 K27M glioma, KRAS-mut ovarian cancer, HER2-mut NSCLC) highlight continued regulatory reliance on molecular stratification.”

More posts featuring Jean-Charles Soria.


