Giuseppe Banna, Consultant Medical Oncologist at Portsmouth Hospitals University NHS Trust, shared a post on LinkedIn:
“Timing, Targets, and the Survival Gap: Lessons from the BR.31 Adjuvant Trial
The publication of the Canadian Cancer Trials Group BR.31 trial in Journal of Clinical Oncology, provides a critical moment for reflection in Thoracic Oncology. While Durvalumab has been a “game-changer” in consolidation settings (PACIFIC and ADRIATIC), it did not meet its primary endpoint in the completely resected NSCLC setting.
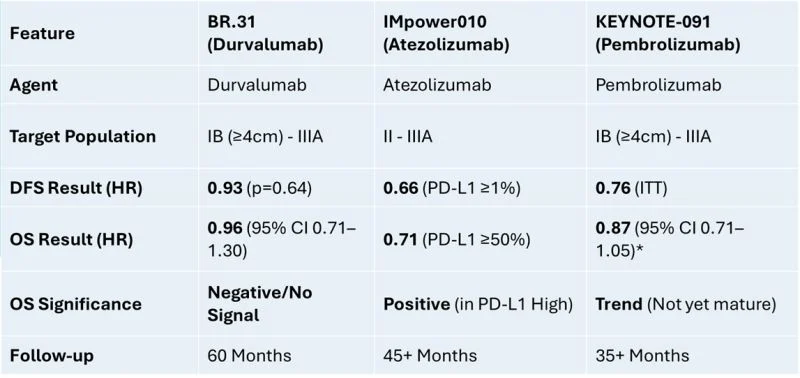
The Data at 60 Months: Despite long-term follow-up, adjuvant Durvalumab showed no significant benefit:
DFS (Disease-Free Survival): HR 0.93 (p=0.64).
OS (Overall Survival): HR 0.96 (95% CI 0.71–1.30).
My Key Takeaway:
1. Inter-class and Intra-class Heterogeneity. This negative result highlights a fundamental question: Are all checkpoints created equal?
– Inter-class differences (PD-1 vs. PD-L1): We are seeing a divergence in the adjuvant setting. While Pembrolizumab (anti-PD-1) showed a DFS benefit regardless of PD-L1 status in KEYNOTE-091, the anti-PD-L1 agents have shown more varied results, with Atezolizumab showing its strongest signal only in high-expressors (IMpower010).
– Intra-class nuances: Even within the same class (anti-PD-L1), molecules behave differently. Durvalumab’s failure in BR.31 contrasts with Atezolizumab’s success in IMpower010. This suggests that binding affinity, dosing schedules, or perhaps the specific interaction with the tumor microenvironment post-surgery varies significantly between these agents.
2. Selection Matters: Unlike IMpower010, which showed a clear OS benefit (HR 0.71) in the PD-L1 ≥50% subgroup, BR.31 saw no separation of curves regardless of PD-L1 expression.
The “Clinical So-What?”: We cannot assume “class effects” when moving from metastatic to adjuvant settings. The failure of BR.31 reinforces that molecule selection and patient stratification (beyond just PD-L1 %) are key.
For now, the adjuvant standard remains firmly with Atezolizumab (for high expressors) or Pembrolizumab.
Understanding these pharmacological nuances is what separates a standard treatment plan from true precision medicine.”
Title: Adjuvant Durvalumab in Completely Resected Early-Stage Non–Small Cell Lung Cancer
Authors: Glenwood D. Goss, Gail E. Darling, Virginie Westeel, Kazuhiko Nakagawa, Bartomeu Massutí, Francesco Perrone, Sue-Anne McLachlan, Jin Hyoung Kang, Yi-Long Wu, Anne-Marie C. Dingemans, Rafal Dziadziuszko, Laurent Greillier, Morihito Okada, Clarisse Audigier-Valette, Shunichi Sugawara, Ernest Nadal, Annamaria Catino, Anne-Claire Toffart, Tetsuya Mitsudomi, Renaud Whittom, Manuel Domine, Nobuyuki Yamamoto, Olivier Molinier, Franck Morin, Penelope A. Bradbury, Martin R. Stockler, Keyue Ding, Christopher J. O’Callaghan

More posts featuring Giuseppe Banna on OncoDaily.


