Eyal Gottlieb, VP of Research at MD Anderson Cancer Center, shared a post on LinkedIn:
“In 2025, several research teams at MD Anderson Cancer Center helped push cancer metabolism from a biochemical curiosity to a central framework for understanding, and potentially treating, metastatic disease. Two recent publications illustrate how metabolic rewiring shapes tumor aggression, systemic physiology, and therapeutic opportunities.
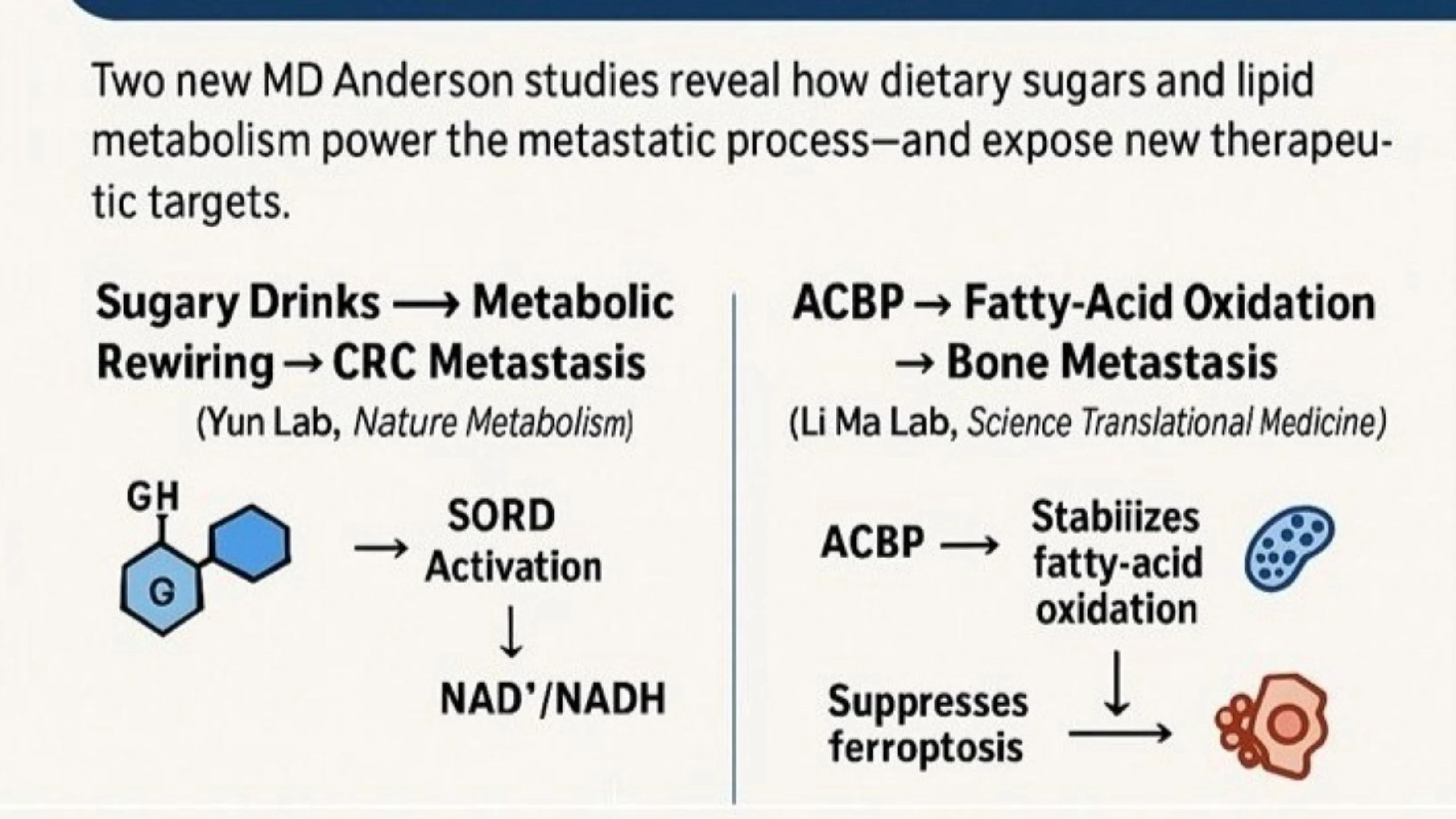
1. Sugary Drinks and Colorectal Cancer Metastasis — A Metabolic Mechanism With Real-World Impact (Nature Metabolism, Jihye Yun Lab)
What do sugar-sweetened beverages do to cancer cells? Jihye Yun’s group uncovered a surprisingly potent metabolic driver of metastasis.
Their Nature Metabolism study demonstrates that a glucose–fructose mixture, mimicking common sugary drinks, reprograms colorectal cancer metabolism through activation of the reverse reaction of the polyol-pathway enzyme SORD (sorbitol dehydrogenase). This shift increases the cellular NAD⁺/NADH ratio, accelerates glycolysis, and funnels carbon into the mevalonate pathway, a promoter of cell migration and metastatic seeding.
Highlights of this work:
- It provides a mechanistic explanation for how dietary sugar can enhance metastatic potential.
- It identifies SORD and mevalonate pathway flux as actionable metabolic nodes.
- It raises the intriguing possibility that statins may dampen this metastasis-promoting circuit.
Punchline: Diet is not just lifestyle; it is a metabolic input that can modulate metastasis through rewired biochemical pathways.
2. Fatty Acid Metabolism as a Driver of Bone Metastasis — ACBP as a Key Regulator (Science Translational Medicine, Li Ma Lab)
The second highlight comes from Li Ma’s group, who uncovered a metabolic determinant of bone metastasis that goes far beyond glycolysis.
Their Science Translational Medicine study shows that acyl-CoA binding protein (ACBP) supports metastatic colonization by tuning fatty-acid oxidation (FAO) and suppressing ferroptosis, the iron-dependent form of cell death. Tumor cells that rely on this ACBP-FAO axis gain a survival advantage in the lipid-rich, high-stress bone microenvironment.
Highlights of this work:
- It places lipid metabolism at the center of metastatic biology.
- It suggests FAO inhibitors or ferroptosis-sensitizing strategies as therapeutic avenues for bone metastatic disease.
- It strengthens a broader theme: metastatic cells survive by re-wiring metabolic stress responses.
Across these studies, a unifying concept emerges:
Punchline: Bone metastasis is not just about stromal signals or niche interactions, it is powered by a lipid-metabolic adaptation that can be targeted.
Metastasis is a metabolic state. Tumor cells that spread must reshape their nutrient use, redox balance, and stress-survival pathways. Those adaptations expose new intervention points.
Whether it is sugary beverages driving pro-metastatic mevalonate flux via SORD, or lipid metabolism sustaining bone-tropic cancer cells through ACBP, these papers highlight how understanding cancer metabolism is reshaping both prevention and therapy.”