Svetlana Nikic, Founder of Precision Oncology Consulting, shared a post on LinkedIn:
“ESMO25, and my last Day 4 Highlights
This year, ESMO hosted the first-ever Tumor-Agnostic Track, where we heard that:
- Every target is now a pan-target (KRAS, NTRK, MSI-H, TMB-high, BRCA/HRD are no longer organ-specific biomarkers)
- The hallmarks of cancer are tumor-agnostic.
- Every drug and target should be evaluated for tissue-agnostic development, with applicability across both solid tumors and hematologic malignancies.
- There should be parallel drug development efforts exploring both tumor-agnostic and tumor-specific agents.
- Collectively, we should aim toward molecular fluency and reducing molecular inequity.
Minimal Residual Disease (MRD) dominated my Day 4 at ESMO25
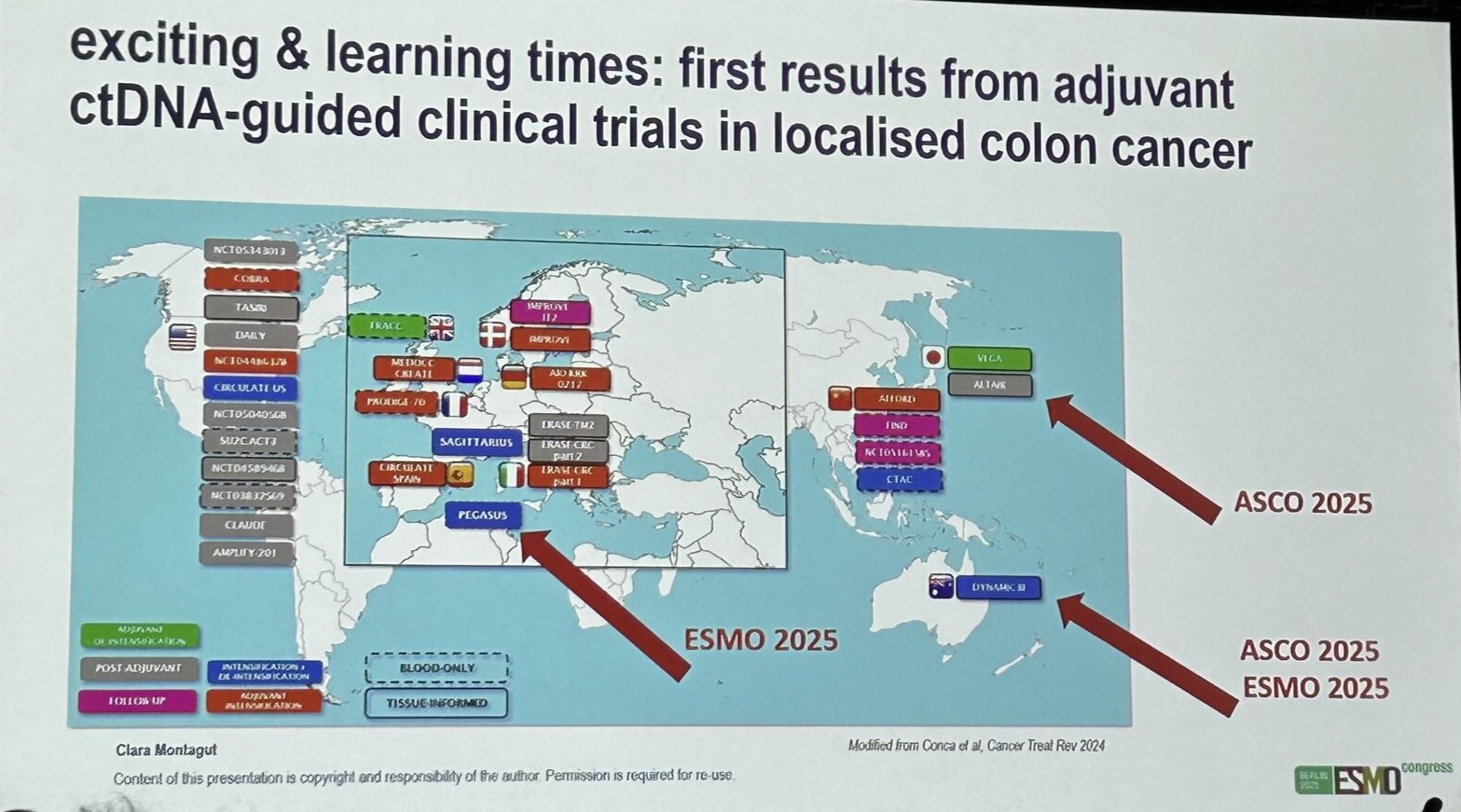
Clara Montagut delivered an excellent overview of the current status of MRD testing in colorectal cancer (CRC):
- CRC is leading the field, with ctDNA MRD being a robust marker of relapse, demonstrated in the first prospective, randomized trial (DYNAMIC II, 2022).
- The number of ongoing ctDNA-guided trials in localized CRC continues to grow.
- It is critical to understand the sensitivity and specificity of your MRD assay:
Escalation trials require high specificity (to avoid overtreatment) and de-escalation trials require high sensitivity (to avoid undertreatment). - ctDNA positivity defines a subgroup of patients with poor prognosis who need more effective, novel therapeutic strategies (beyond chemotherapy escalation).
- For de-escalation in ctDNA MRD-negative patients, it is essential to use a highly sensitive assay to minimize false negatives.
Key practical recommendations:
- Blood draw for MRD assesement should be done at least 2 weeks post-surgery.
- Double testing (two separate blood draws within the MRD window) can improve sensitivity and specificity.
- Consider site-specific differences in ctDNA shedding.
- Longitudinal ctDNA testing may be essential to optimize adjuvant therapy (sustained ctDNA clearance is associated with better DFS).
First prospective randomized MRD-guided trial in Muscle-Invasive Bladder Cancer (MIBC): IMvigor011 (Congrats to the Natera team!)
Key findings:
- Adjuvant atezolizumab led to both DFS and OS benefit vs placebo.
- The timing of the first ctDNA-positive test matters — early positivity (59.2%) indicated higher risk compared to late positivity (40.8%).
- This trial advances the concept from “treat most” to “treat those with molecular evidence of disease.”
Outstanding Questions
- Do we need to further improve ctDNA sensitivity?
- What is the cost-effectiveness of ctDNA-guided vs. treat-all strategies in MIBC?
- What is the optimal ctDNA approach — tumor-informed or tumor-naïve?
If you find this type of insight useful, please like and share.
Also, watch out for a comprehensive ESMO25 Takeaways, which I’ll publish on my blog in a few days.”
More posts featuring Svetlana Nikic.


