The ESMO 2025 Congress is a major global oncology event organized by the European Society for Medical Oncology (ESMO).
It is taking place at Messe Berlin in Berlin, Germany, from October 17 to 21, 2025. The congress features a comprehensive scientific and educational program designed to foster exchange and debate in translational cancer science, showcasing potentially practice-changing data, and stimulating multidisciplinary discussions to improve cancer treatment options.
Tuğba Başoğlu, Associate professor at Memorial Healthcare Group Göztepe Onkoloji Merkezi, shared Highlights from ESMO2025 on X:
“HER2-mutant NSCLC: Zongertinib showing promise across lines of therapy!
Accelerated FDA approval based on Phase 1b BEAMION-LUNG-1:
ORR: 75% DoR ≥6mo: 58%
Intracranial ORR: 41%
Now at ESMO2025 – First-line data unveiled:
ORR (BICR): 77%
PFS & 6-mo DoR>80%”
“Both HER2-targeted TKIs are coming in hot! CNS efficacy takes center stage at ESMO25.
Zongertinib (Beamion LUNG-1)
- Brain ORR: 41%
- FDA approved, 1L trial ongoing
Sevaberitinib (SOHO-1)
- Brain ORR: 88%
- Ph3 (SOHO-2) launched vs chemo-IO.”
The phase II NorthStar trial shows that adding local consolidative therapy (LCT) to osimertinib significantly improves PFS in EGFR-mutant metastatic NSCLC:
- 25.3 vs 17.5 months
- HR 0.66 | p=0.025
- Close monitoring advised, esp. with RT.”
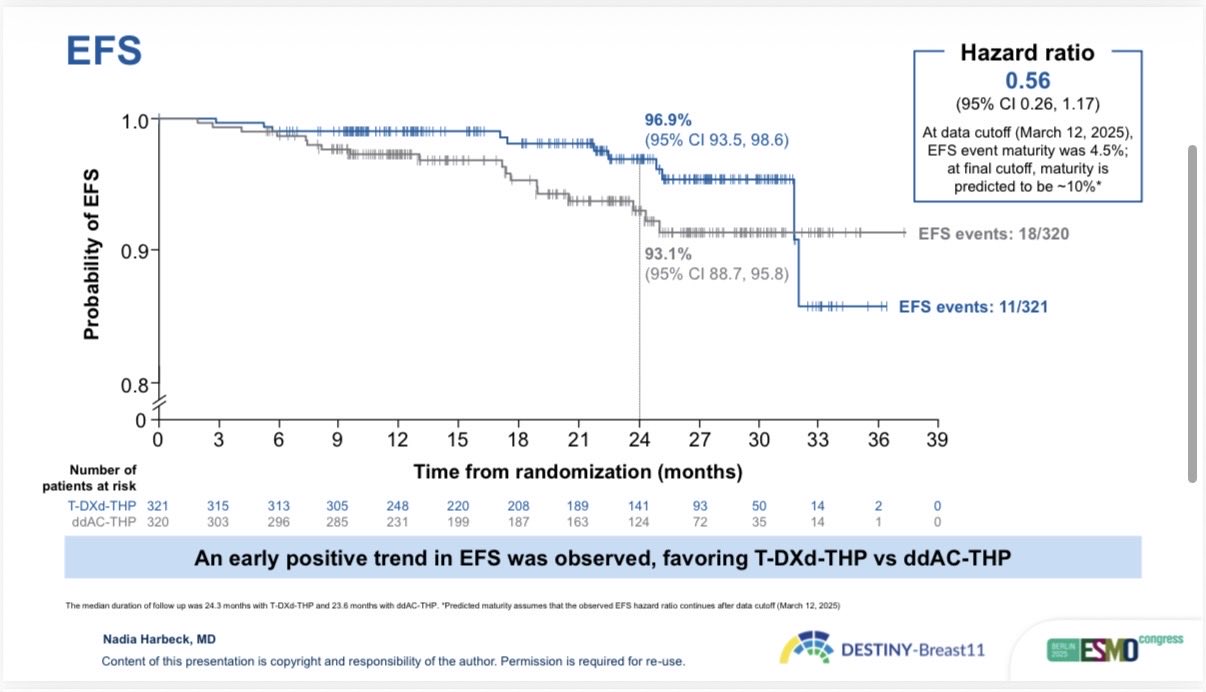
“ESMO25 BREAKING: DESTINY-Breast11 shows T-DXd-THP achieves highest ever pCR (67.3%) in high-risk HER2+ eBC.
Statistically significant improvement vs ddAC-THP (Δ11.2%, p=0.003).
EFS trend favors T-DXd-THP (HR: 0.56).
A potential new neoadjuvant standard.”

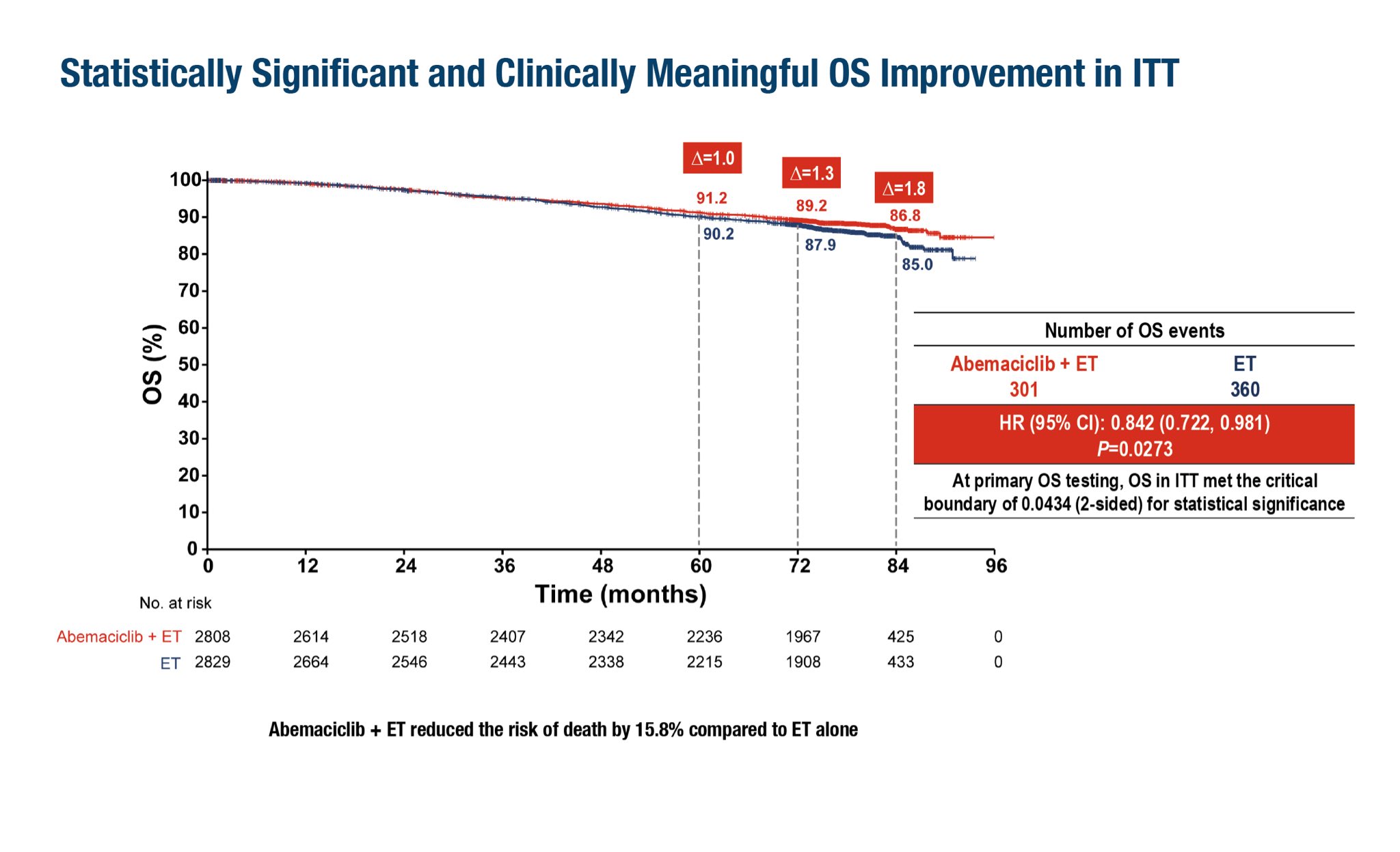
7-year OS benefit with abemaciclib(monarchE)
15.8% ↓risk of death | OS: 86.8% vs 85%(HR 0.84, p=0.027)
5-year relapse reduction with ribociclib(NATALEE)
28.4% ↓ risk of IDFS: 85.5% vs 81.0% (HR 0.72, p<0.0001)
CDK4/6inh are reshaping adj therapy in BC.”
Adoptive cell therapy is stepping into the solid tumors.
TIL/TCR (J. Haanen)
CAR-T (S. Guedan)
NK-based therapies (J. Miller)
Challenges?
- Antigen loss
- Immunosuppression
- T cell exhaustion
A future where cell therapy reshapes solid tumor care is coming – faster than we think.”
“ESMO25 BREAKING: DESTINY-Breast11 shows T-DXd-THP achieves highest ever pCR (67.3%) in high-risk HER2+ eBC.
Statistically significant improvement vs ddAC-THP (Δ11.2%, p=0.003) EFS trend favors T-DXd-THP (HR: 0.56).
A potential new neoadjuvant standard.”

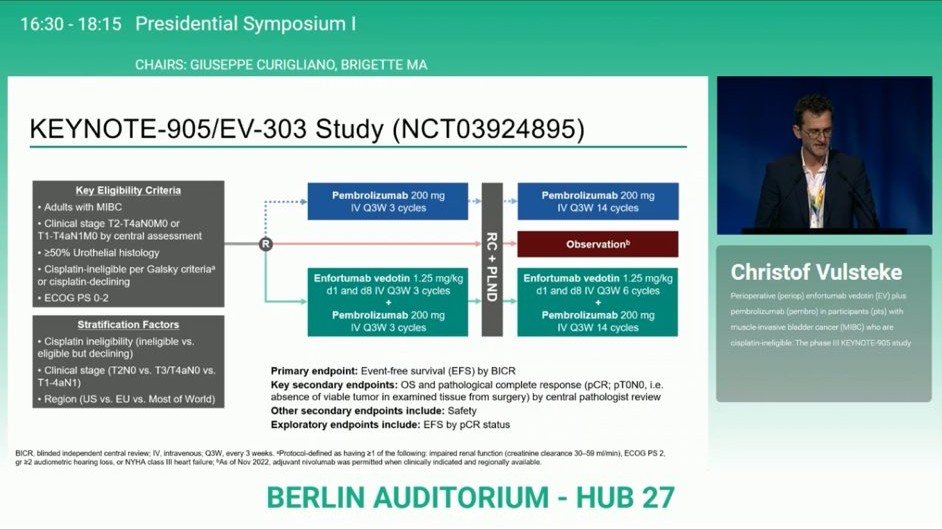
“Unveiled to applause! KEYNOTE-905.
LIVE ESMO25: Perioperative EV + pembro delivers game-changing results in cisplatin-ineligible MIBC:
- EFS HR: 0.40
- OS HR: 0.50
- pCR: 57.1% vs 8.6%
First-ever phase 3 trial showing periop benefit in this setting. A potential new standard of care is on the horizon!”
(PRROC) – ENGOT-ov65/KEYNOTE-B96 Pembrolizumab + weekly paclitaxel ± bevacizumab shows significant PFS & OS benefit in platinum-resistant recurrent ovarian cancer.
First checkpoint inhibitor to improve OS in PRROC!”

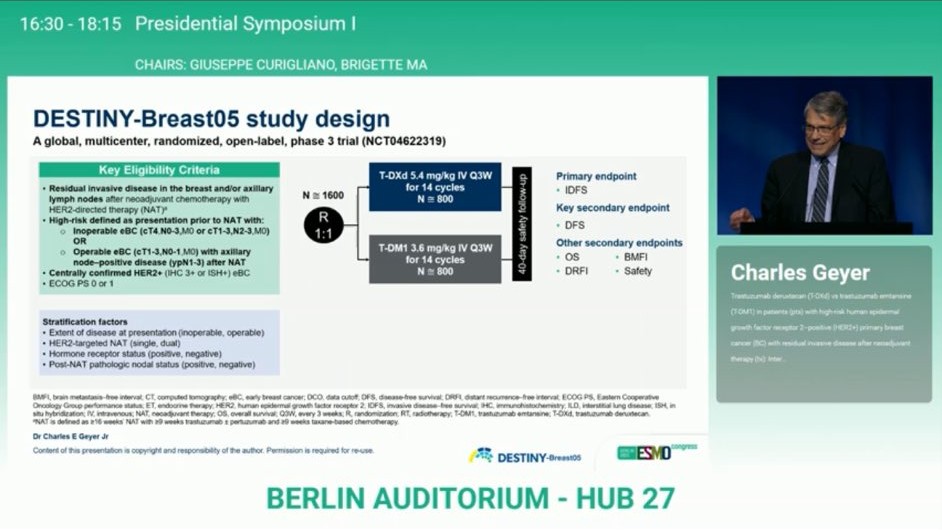
“ESMO25 Continues to Impress: Game-Changing Data from DESTINY-Breast05!
T-DXd vs T-DM1 in high-risk HER2+ early breast cancer post-neoadjuvant therapy:
- 3-yr IDFS: 88.7% vs 77.8%.
- HR: 0.47 → 53% reduction in recurrence/death.
- Stat sig. benefit across key subgroups.
- A practice-changing moment?”
“LIVE from ESMO25: HORIZON-Breast01
New hope for HER2+ metastatic breast cancer!
SHR-A1811 (trastuzumab rezetecan) shows superior PFS vs pyrotinib + capecitabine:
mPFS: 30.6 vs 8.3 months
ORR: 81.7%
Low ILD (2.8%)
HORIZON-Breast01 may be practice-changing.”
“LIVE ESMO25 UPDATE | DESTINY-Breast09 subgroup data is here!
T-DXd + pertuzumab > THP for PFS across all subgroups:
- HR+ / HR−
- De novo / Recurrent
- PIK3CA mutated / wild type
No new safety signals
ILD manageable across subgroups
T-DXd + P shows promise as 1L standard in HER2+ a/mBC
Stay tuned – full analysis coming soon with DESTINY-Breast05, 09 and 11 together!”
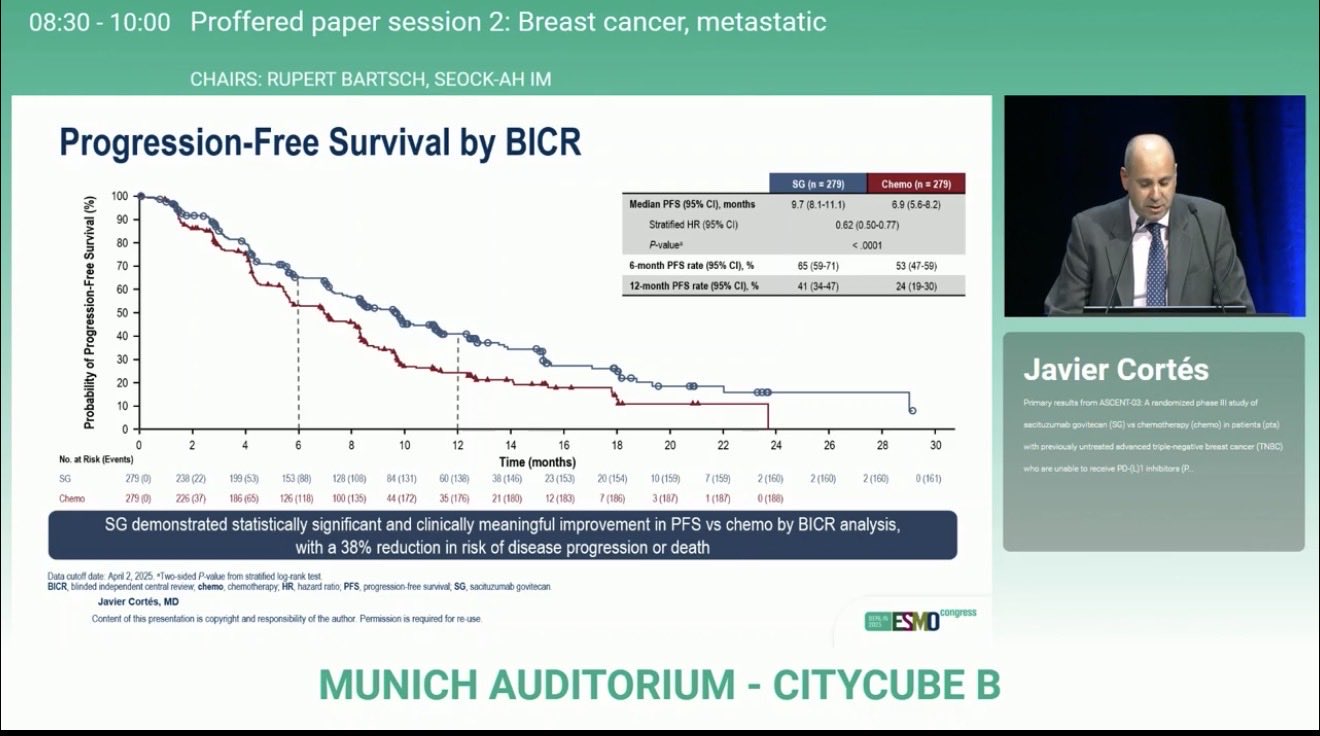
“ASCENT-03 shaking up TNBC treatment at ESMO25.
SG > chemo in 1L mTNBC (PD-L1 ineligible).
Median PFS: 9.7 vs 6.9 mo.
Fewer AEs, lower discontinuation.
Durable responses across subgroups.
Another practice-changing moment on the ESMO stage!”
“TROPION-Breast02 delivers standing ovation-worthy results at ESMO25!
1L Dato-DXd > ICC chemo in mTNBC
Dual primary endpoints met:
- mPFS: 10.8 vs 5.6 mo → HR 0.57
- mOS: 23.7 vs 18.7 mo → HR 0.79
- ORR: 62.5% vs 29.3%
Safety manageable despite double treatment duration A strong contender for new 1L standard in PD-L1 ineligible mTNBC.”
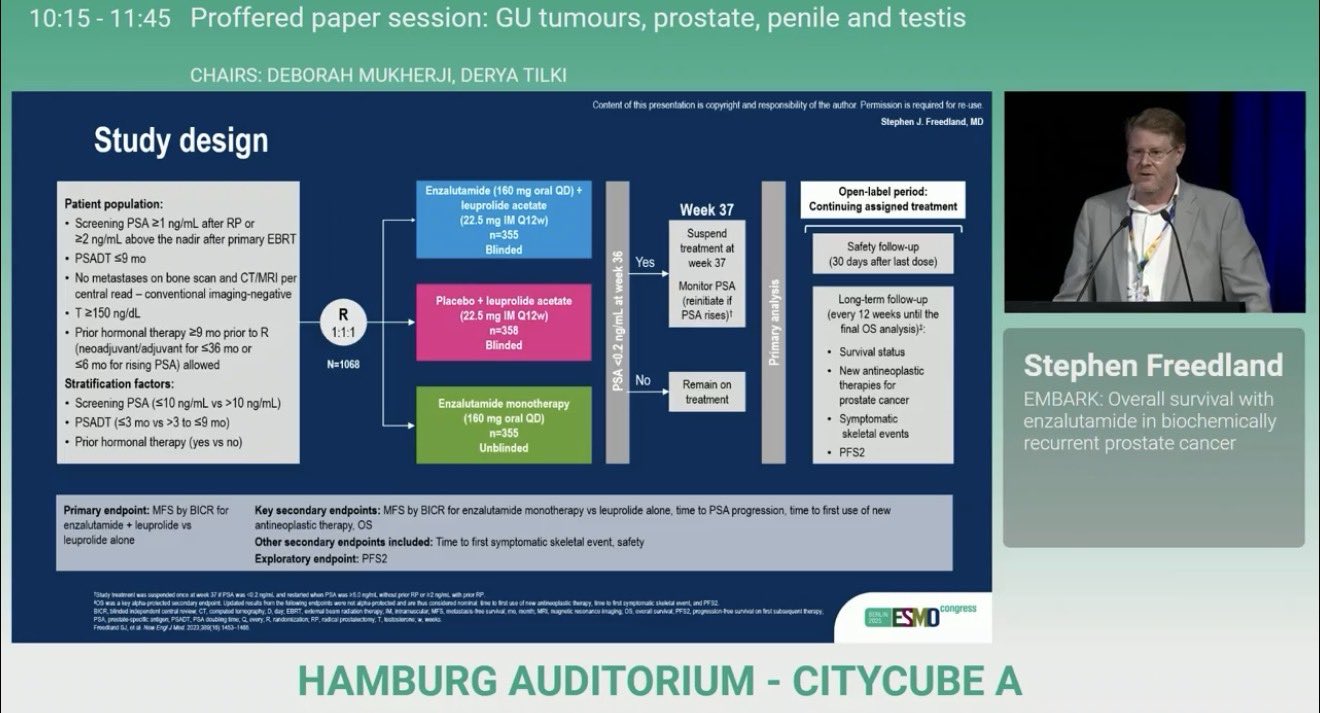
“Breaking from ESMO25 | EMBARK Trial Update
Enzalutamide + ADT significantly improves overall survival in high-risk biochemically recurrent Prostate Cancer vs ADT alone!
- HR: 0.597 8-year
- OS:
• Enza combo: 78.9%
• ADT alone: 69.5% - Risk of death reduced by >40%
- No OS benefit with enzalutamide monotherapy
- No new safety signals
- Major win for patients – new standard of care on the horizon!
Presented by Dr Stephen Freedland at ESMO2025 GU Tumors Session.”
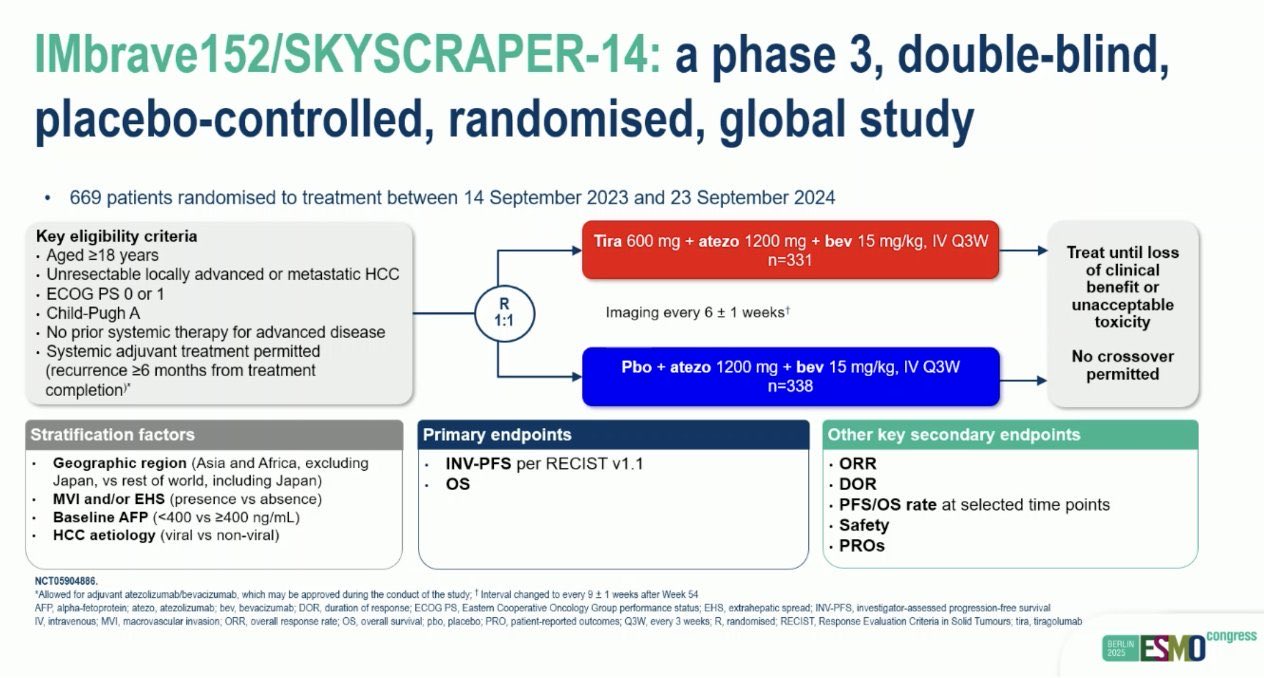
“Negative trial
Phase III IMbrave152/SKYCRAPER-14:
Tiragolumab + Atezolizumab + Bevacizumab vs Atezolizumab + Bevacizumab in 1L unresectable/metastatic HCC
- Primary endpoint (INV-PFS) not met
- 8.3 vs 8.2 mo | HR 0.97 (95% CI 0.8–1.2)
- ORR: 30% vs 26%
- No added benefit over current SOC
- IMbrave150 remains the standard of care in 1L HCC.
Safety acceptable, but efficacy flat.”
“LIVE ESMO25 | Is Lutetium Moving Up Front in Prostate Cancer?
The PSMAddition trial lays the groundwork for future use of Lu-177 in earlier treatment lines—not just in resistant disease. While data are promising, mature OS results are awaited. Let’s watch the space as evidence evolves.”
You Can Also Read: PSMAddition at ESMO 2025: ¹⁷⁷Lu-PSMA-617 Plus ADT and ARPI Improves PFS in PSMA-Positive mHSPC

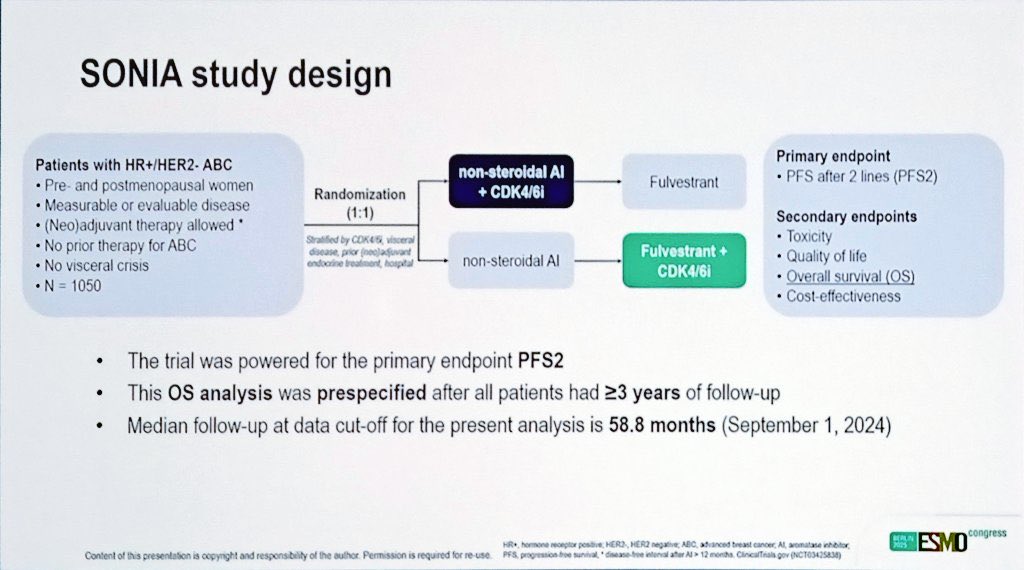
“LIVE ESMO25 | Updated SONIA Trial.
No OS benefit for 1L vs 2L CDK4/6i in HR+/HER2– advanced breast cancer overall.
But post-hoc data suggest:
In selected postmenopausal pts with low disease burden, starting with AI alone may be reasonable—limiting early toxicity & cost.
Tailored treatment matters.”
You Can Also Read:
ESMO 2025 Day 1 Highlights Not to Miss


