Emil Lou, Professor with Tenure at University of Minnesota, shared a post on LinkedIn:
“Continuing these posts as thoughts come to mind: More reflections here and there on what was presented earlier this month at GI26, the American Society of Clinical Oncology (ASCO) Gastrointestinal Cancers Symposium:
In the past decade, we have seen relative leaps and bounds in identification of actionable target biomarkers in gastroesophageal cancers… closely followed up by proof of execution that targeting that markers can make a difference in progression-free and overall survival of affected patients, And all the while bringing those targeted therapies up from third-line and beyond, all the way to the frontline setting. This means we need to have Fast turnaround of testing to get the results in the hands of oncologists and patients alike, for informed clinical decision-making in real time.
The HERIZON-GEA-01 trial examined use of Zanidatamab (a bispecific monoclonal antibody for HER2) with or without addition of Tislelizumab (a IO PD-1 inhibitor) for treating patients with HER2+ Metastatic Gastroesophageal Adenocarcinoma in the frontline setting.
Patient Population:
Previously untreated patients with unresectable locally advanced or metastatic HER2-positive gastroesophageal adenocarcinoma (GEA), HER2 IHC 3+ or IHC 2+/ISH+. ≈914 participants.
Treatment Arms:
- Zanidatamab + chemotherapy + tislelizumab (PD-1 inhibitor)
- Zanidatamab + chemotherapy
- Trastuzumab + chemotherapy (standard of care control)
Key Outcomes:
Progression-Free Survival (PFS):
- Zanidatamab arms (both with and without tislelizumab): median PFS ~12.4 months vs 8.1 months with trastuzumab/chemo.
Overall Survival (OS):
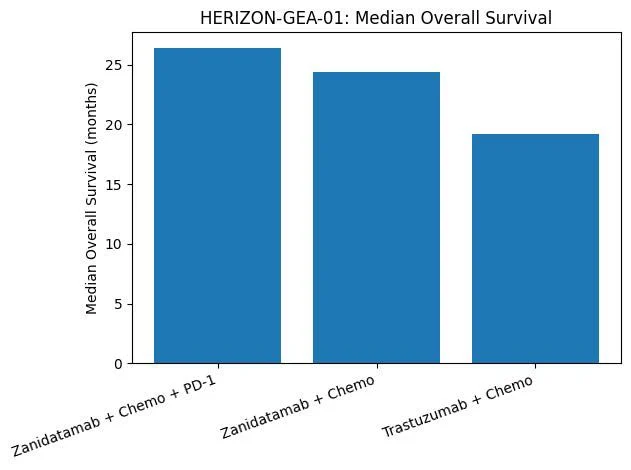
- Zanidatamab + tislelizumab + chemo: median OS ~26.4 months vs 19.2 months with trastuzumab + chemo (statistically significant).
- Zanidatamab + chemo alone showed an OS trend above control (~24.4 months) but did not reach statistical significance in this interim analysis.
Response Rates:
- Complete Response (CR): ~19.6% (zanidatamab/tislelizumab), ~17.1% (zanidatamab only), ~11.0% (trastuzumab).
- Partial Response (PR): ~51.1% (zanidatamab/tislelizumab), ~52.5% (zanidatamab), ~54.8% (trastuzumab).
Safety:
- Grade ≥3 adverse events were more common with zanidatamab + tislelizumab (≈72%) than with control (~59%), with diarrhea, hypokalemia, and anemia frequent.
Interpretation: - These results suggest zanidatamab-based regimens may become a new first-line standard for HER2+ metastatic GEA, exceeding outcomes historically seen with trastuzumab combos.”
Title: HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma
Authors: Josep Tabernero, Lin Shen, Elena Elimova, Geoffrey Ku, Tianshu Liu, Kohei Shitara, Xiao Lin, Lisa Boyken, Huiyan Li, Jonathan Grim, Jaffer Ajani
Read the Full Article in Taylor and Francis.

More posts featuring Emil Lou.


