Brian Lawenda, Radiation Oncologist at Advocate Radiation Oncology, shared a post on Substack:
“Why spend a decade inventing a new drug when the cure might already be sitting on a pharmacy shelf, or in a molecule AI designs from scratch in seconds? Enter the era of AI-driven oncology.
A New Imaginative Frame for Cancer Treatment
Imagine you are a cancer patient facing a diagnosis that feels like a death sentence. Your doctor outlines options that sound painfully familiar: experimental therapies that might take years to mature, or standard treatments with grueling side effects and uncertain outcomes.
Now imagine something radically different. Imagine that, hidden in the vast sea of already-approved medications, or in a molecule AI designs from scratch in seconds, is a lifeline tailored precisely to your tumor. And imagine we could find that lifeline not in a decade, but in days.
This isn’t science fiction. It is the dawn of AI-powered oncology.
From Tool to Agent: The Rise of Intelligent Discovery Systems
For decades, AI in biomedicine functioned as a glorified calculator. It was a tool to speed up spreadsheets or optimize search results. In 2025, that changed. AI became an agent.
Unlike a calculator, which waits for you to press buttons, an agent acts on its own. It generates hypotheses, tests them, and learns from the results. This is the difference between a soloist and an orchestra.
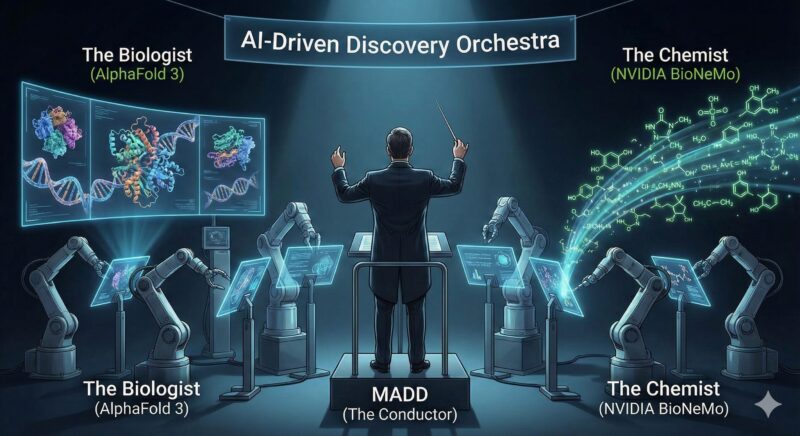
The Biologist: AlphaFold 3 (The Mapmaker)
To treat cancer, you first have to understand the shape of the enemy. Proteins are the microscopic machines that drive biology; if they fold into the wrong shape, disease follows. For 50 years, predicting these shapes was biology’s “grand challenge.”
Then came Google DeepMind, the same AI lab that built the system to beat human champions at the game of Go. Their tool, AlphaFold 3, solved the problem. It does not just predict protein structures; it simulates how they interact with DNA and other molecules. It effectively gives researchers a 3D map of the “locks” on a tumor cell, turning targets that were once invisible into clear, actionable data.
The Chemist: NVIDIA BioNeMo (The Architect)
Once AlphaFold identifies the “lock,” you need a key to fit it. Enter NVIDIA. Best known for making the chips that power video games and ChatGPT, NVIDIA has pivoted to biology.
Their platform, BioNeMo, functions like a “ChatGPT for biology.” But instead of writing poems, it writes chemical code. It uses generative AI to dream up entirely new drug-like compounds that do not exist in nature, optimizing them for safety and effectiveness before a physical lab ever synthesizes them.
The Conductor: MADD (The Manager)
Having a Mapmaker and an Architect isn’t enough; you need them to talk to each other. This is where MADD (Multi-Agent Drug Discovery) comes in.
Unveiled in late 2025 by researchers at ITMO University, MADD acts as the project manager. It breaks a complex command (like “find a cure for this lung cancer variant”) into tasks and assigns them to specialized sub-agents:
- The Decomposer: Breaks the complex request into logical steps.
- The Orchestrator: Selects the right AI tools to generate and validate molecules.
- The Summarizer: Compiles the data into a final, clinician-ready report.
While the system was initially benchmarked on seven diseases, including Alzheimer’s and Parkinson’s, its application in oncology has been particularly promising. In one weekend, it coordinated these agents to screen over 3 million candidate molecules—a scale of trial-and-error that would take human teams years to replicate in the physical world. While MADD is a prime academic example, this “conductor” model is rapidly becoming the industry standard, with major U.S. players like NVIDIA and Google building similar frameworks to orchestrate their own discovery pipelines.
Reality Check: Design Isn’t Validation AI can design promising molecules in hours. But biology remains physical. A perfect binding in silico (on a computer) may fail in vivo (in a living body) due to metabolism or unforeseen toxicity. We have compressed the design phase, but wet-lab validation, animal models, and clinical trials remain essential. The challenge ahead is accelerating these physical phases without compromising safety..
Rediscovery, Not Reinvention: The Power of Repurposing
Some of the most exciting breakthroughs in oncology may come not from new molecules, but from old ones that are reimagined.
There are thousands of drugs already approved by the FDA for conditions ranging from diabetes to arthritis. Sometimes, these drugs have hidden side effects that fight cancer, a phenomenon known as “drug repurposing.” In the past, finding these was luck (like discovering Viagra began as a heart medication). Today, AI systems scour the molecular data of every approved drug to find these matches systematically.

Repurposing bypasses the slowest parts of development because we already know these drugs are safe for humans. Where humans might test a few hundred compounds per year, AI systems screen millions virtually. What once took a decade now begins in a weekend.
Case Study: STAT3 and the Virtual Pipeline
In the ITMO study, the MADD system was tasked with finding inhibitors for STAT3, a protein often linked to aggressive lung cancer. Over the course of days, the system screened massive libraries and identified thousands of candidates. Notably, some of these molecules were predicted to perform better than existing experimental drugs.
The implication is clear: hit discovery and lead optimization can occur at machine speed. In cancers where time is everything, this is not an incremental gain. It is a survival advantage.
Reality Check: Repurposing Still Faces Bottlenecks
Repurposed drugs must still navigate regulatory pathways. Because these drugs are often cheap generics, pharmaceutical companies may lack the financial incentive to fund expensive trials for them. AI may radically compress the science, but the economics remain tricky.
From Population Medicine to N-of-One Therapy
The logical endpoint of AI-driven discovery is “N-of-one” medicine: treatments customized not for populations, but for you.
This relies on Digital Twins, highly detailed computer models of a single patient’s biology. By feeding an AI your genetic data, tumor profile, and immune history, doctors could simulate how your body reacts to a drug before you ever take it.
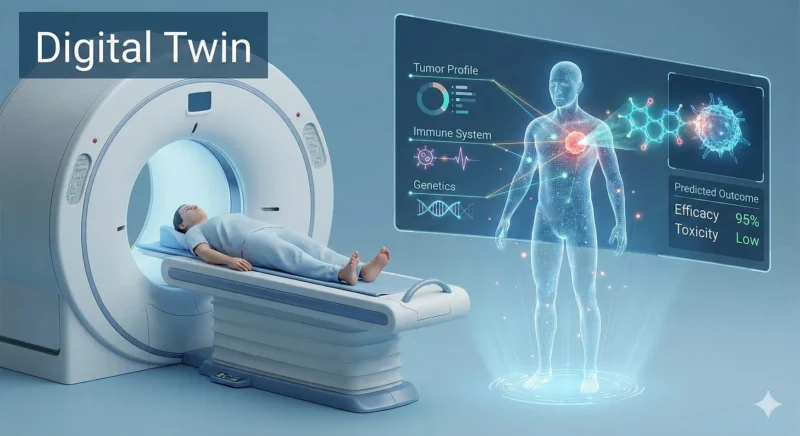
Instead of asking “Does this drug work for the average patient?” we ask: “What’s the optimal therapy for this person, today?”
Reality Check: Personalized Medicine Isn’t Plug-and-Play
Even with perfect simulations, biology is unpredictable. The microbiome, tumor mutations, and immune responses are incredibly complex. Regulatory systems (like the FDA) are built to approve drugs for large groups, not single individuals. The science is advancing faster than the legal framework.
Clinical Trials, Reimagined
As AI moves closer to simulating human physiology with high fidelity, the clinical trial process itself transforms. Instead of 5-year studies requiring thousands of patients, we could see rapid, virtual-first validations followed by smaller, highly monitored human trials.
The trial does not disappear. It shifts. It moves from a massive, slow statistical hunt to a precise, model-based validation.
The Far Horizon: Real-Time, On-Demand Drug Design
As AI converges with robotics and precision manufacturing, the supply chain itself could decentralize. Imagine “pharmacies” that do not just store pills, but synthesize custom therapies on-site using robotic chemistry, tailored to the AI’s design for your specific condition.
Not a dream. A trajectory.
Strategic Implication: We Are Entering the AI-First Drug Discovery Era
This is not hype. AI is no longer an assistant. It is the lead actor in early discovery. The firms, labs, and regulators that adapt fastest will shape the future of oncology.
Final Reality Check: Speed Must Serve Safety
AI is rewriting the speed of science. But speed without validation is just acceleration into a wall. We must build the systems, governance, and ethics to match the technology.
Done right, this is not just a new pipeline. It is a new contract with biology itself.”
More posts featuring Brian Lawenda.


