Ashish Kamat, Professor and Director of Urologic Oncology Fellowship at MD Anderson Cancer Center, shared a post on X about a paper he co-authored with colleagues published in the European Urology Oncology:
“Our latest European Urology Oncology consensus from the International Bladder Cancer Group (IBCG) provides updated recommendations for Intermediate-Risk NMIBC – a group representing ~50–60% of all NMIBC yet often inconsistently managed.
We define IR-NMIBC precisely and outline evidence-based strategies for care and clinical trials.
Definition and Risk Stratification
- IR-NMIBC = Low-grade (G1–G2) Ta or T1 tumors that are multifocal, >3 cm, or recurrent
- All High-grade (G3) tumors → High-risk.
- The validated IBCG IR Score enables tailored, risk-based management rather than a one-size-fits-all approach.
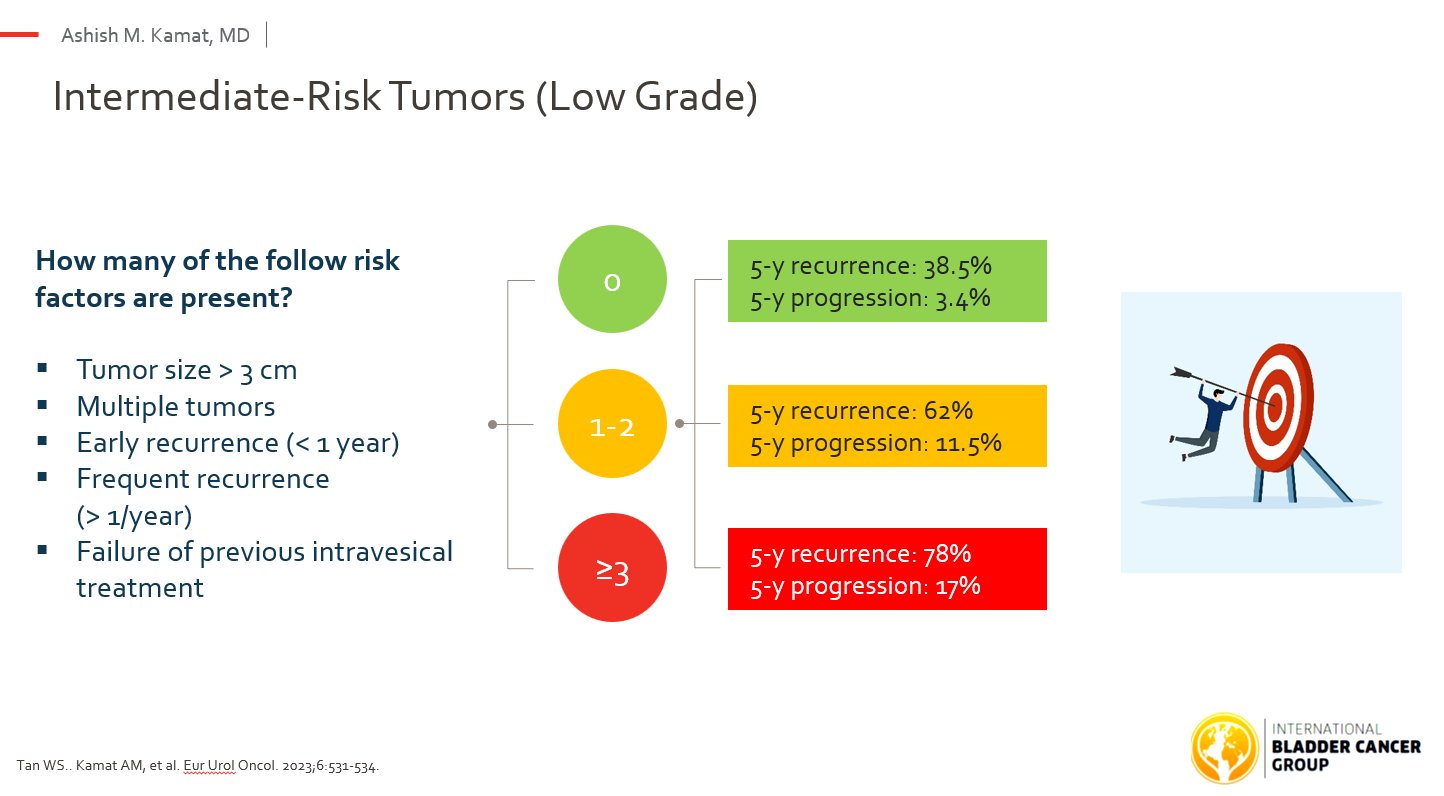
Clinical Management
- Peri-operative intravesical chemotherapy reduces recurrence by ≈ 35% — should be standard.
- Re-TURBT is unnecessary after complete resection of LG Ta.
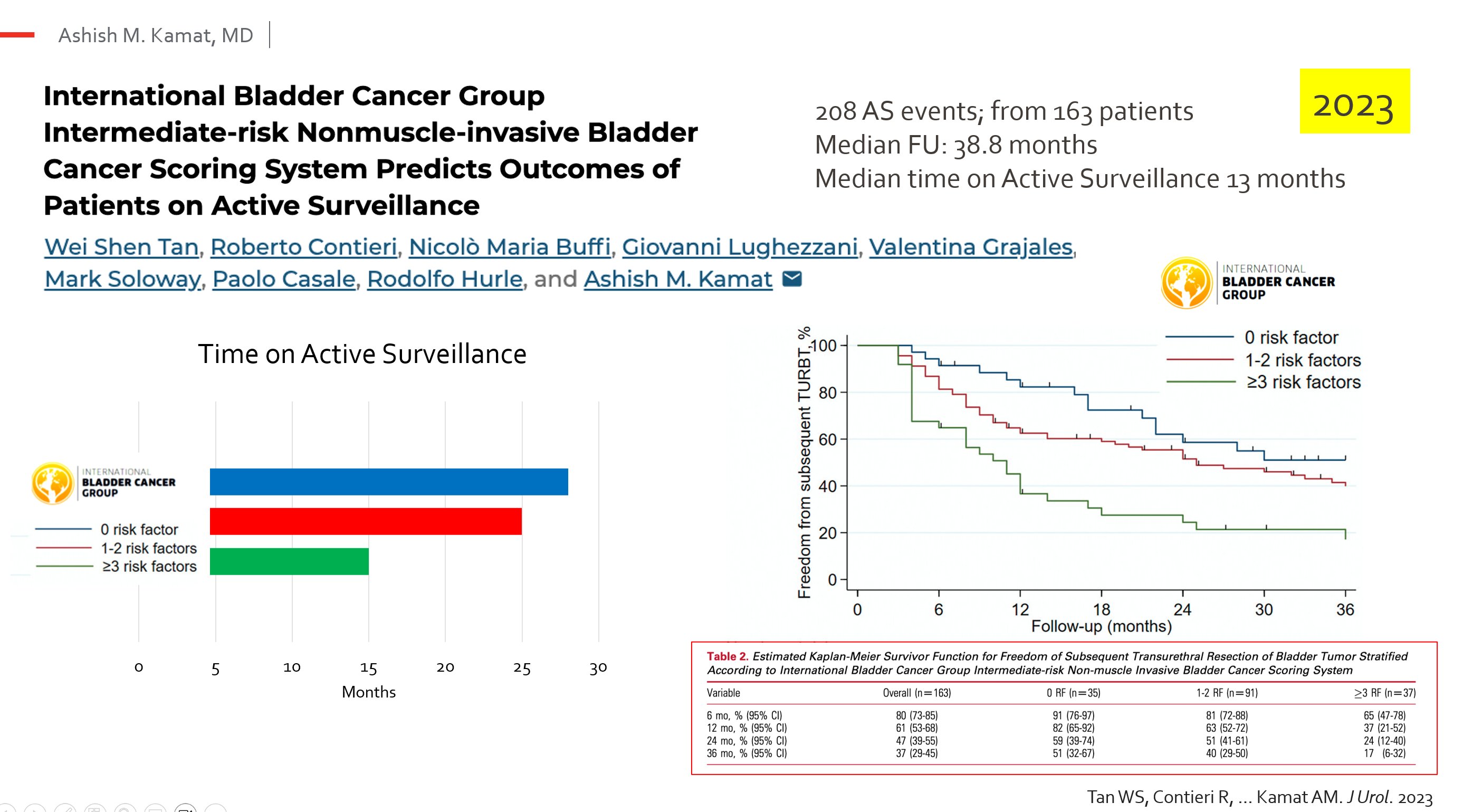
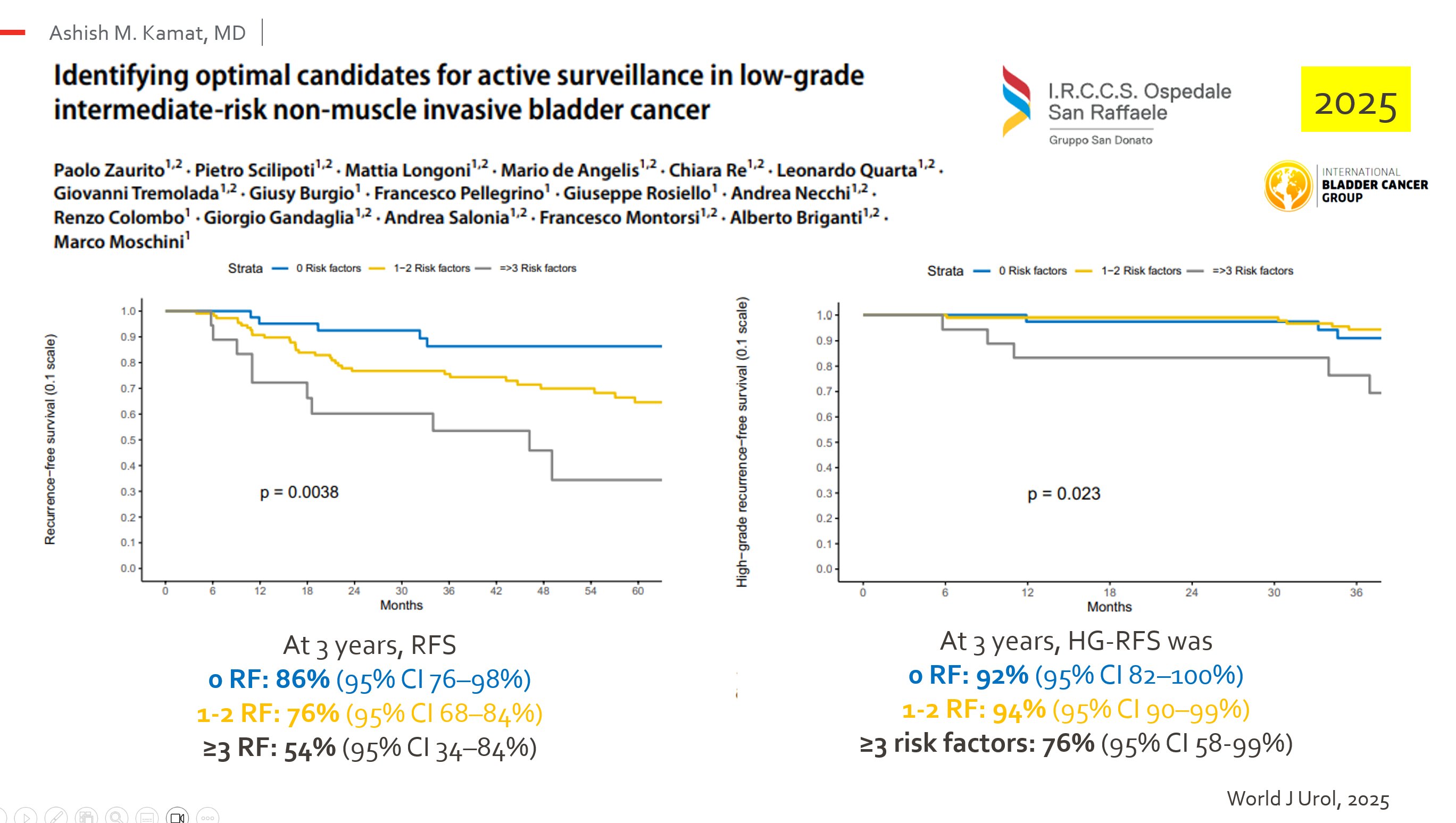
- Active surveillance is safe for patients with ≤ 1 risk factor and negative cytology.
- Office-based ablation suitable for ≤ 5 papillary lesions < 1 cm.
Focus: minimize overtreatment and procedure-related morbidity.
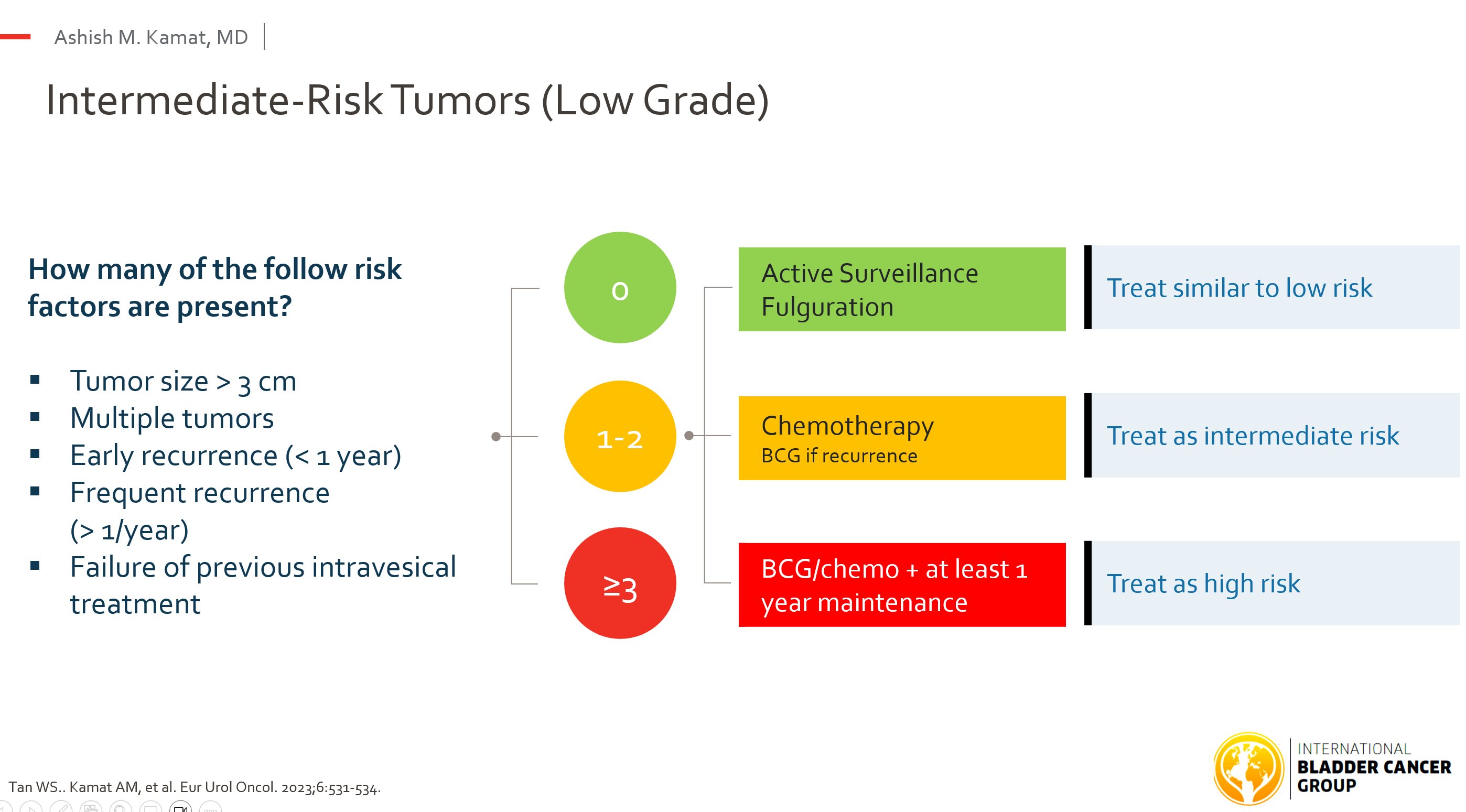
Adjuvant Therapy Framework
- Prefer chemotherapy > BCG, especially during shortages.
- 0 factors: single peri-operative dose.
- 1–2 factors: add 6-wk induction chemo ± maintenance (1 yr) or BCG.
- ≥3 factors: induction + 1 yr maintenance BCG.
- Recurrent/highest-risk: consider dual chemo, chemohyperthermia, or clinical trials over cystectomy.
Goal: preserve the bladder without compromising oncologic safety.
Surveillance and Trials
- Cystoscopy at 3 mo → q6 mo × 2 yrs → annually if no recurrence.
- Routine cytology / enhanced cystoscopy not needed for most.
- Trials should use risk-stratified controls, 10% effect size for 2-yr RFS, and include patient-reported QoL endpoints.
This consensus brings clarity to IR-NMIBC – aligning practice, reducing burden, and advancing trial design worldwide.”
Sabine D. Brookman-May, Senior Vice President and Therapeutic Area Head of Urologic Oncology at Aura Biosciences, shared this post, adding:
“Excellent – thank you for sharing, Ashish M. Kamat. Great that IBCG drives the work on better defining risk categories and management of IR NMIBC, including the need to reduce over-treatment.”
Title: Intermediate-risk Non-muscle-invasive Bladder Cancer: Recommendations for Definitions, Risk Stratification, Management Strategies, and Clinical Trial Design from the International Bladder Cancer Group
Authors: Roger Li, Patrick J. Hensley, Marko Babjuk, Laura Bukavina, Sarah P. Psutka, Seth P. Lerner, Michael A. O’Donnell, Yair Lotan, Kelly K. Bree, Joan Palou Redorta, David J. McConkey, Byron H. Lee, Paramanathan Mariappan, Laura S. Mertens, Mark S. Soloway, Robert S. Svatek, Wei Shen Tan, Stephen B. Williams, Shilpa Gupta, Roger Buckley, Ashish M. Kamat
You can read the Full Article in the European Urology Oncology.

More posts featuring Ashish M. Kamat.


