Amol Akhade, Senior Consultant at Fortis Hospitals Mumbai, shared a post on X:
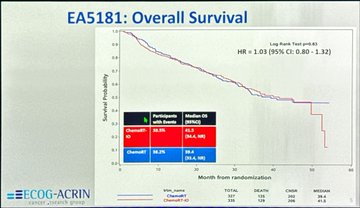
“EA5181 Trial
In unresectable stage III NSCLC, adding concurrent durvalumab to CRT (vs CRT alone → durva consolidation) showed:
- No OS benefit – HR 1.03 (95% CI 0.80–1.32)
- No PFS benefit – HR 1.05 (95% CI 0.86–1.29)
- No change in recurrence or ORR
- PACIFIC remains the standard: CRT 1 yr durvalumab consolidation.”
More from Amol Akhade on OncoDaily.


