Ben Derman, Assistant Professor at University of Chicago, shared a post on X:
“The final results of the CANOVA study (Ven-dex vs Pom-dex) is a lesson in trial design.
Focused on t(11;14) only; randomized 1:1
Pom-Dex as comparator; imbalance in patients withdrawing from pom-dex. Suspect because suboptimal control arm (more on that below).
Primary endpoint = PFS. While curves separate, it is not significant. Let’s see why….
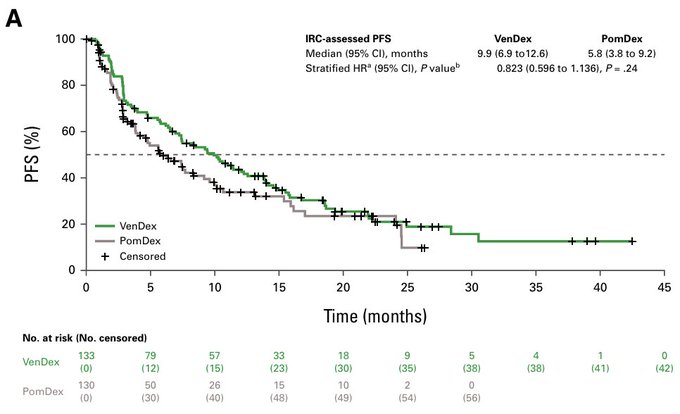
The theory here is that more patients in pom-dex were taken off study because of sub-optimal response (before progression) or better options. So when investigators adjusted for this (event-free survival and time to next treatment), there is an advantage for ven-dex. Unfortunately, this is not statistically sound enough for any approval. And so…we still don’t have an approval for venetoclax in myeloma. Read more.
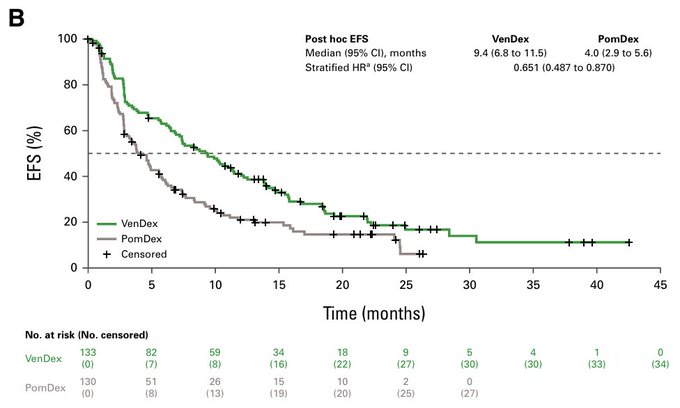
The moral of the story is to design trials with standard-of-care arms that are actually SOC. That this doesn’t happen is not entirely the fault of sponsors.
Take the 1-3 prior line space right now. Most options being investigated are Dara-Pd, Dara-Vd, Elo-Pd, and Kd. Sometimes Dara-Kd may be used as well. Most patients are anti-CD38-refractory making these regimens less attractive.
Regulators won’t accept regimens like VPd, KPd, KCd, Dara-KPd. So when these types of phase 3 trials read out, I suspect they will be largely useless to us as a field. We will have moved on to bispecifics, CAR T, Bela-Vd/Pd, and we won’t be able to contextualize the findings.”
More posts featuring Ben Derman.


