Tanja Obradovic: FDA just announced withdrawal of the approval for BridgeBio Pharma Truseltiq
Tanja Obradovic shared a post on LinkedIn:
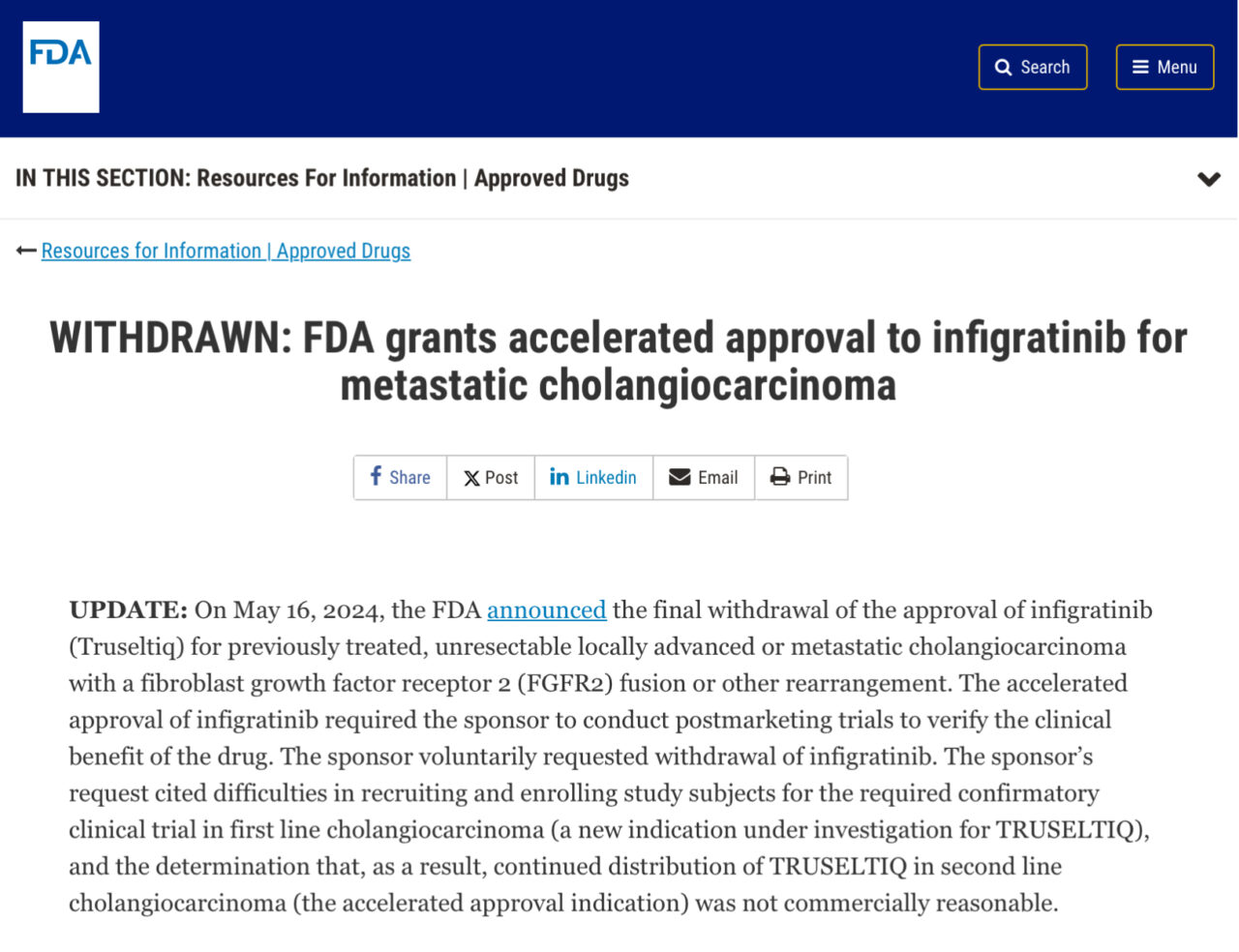
“FDA just announced withdrawal of the approval for BridgeBio Pharma Truseltiq (infigratinib) for previously treated, unresectable locally advanced or metastatic cholangiocarcinoma who harbor an FGFR2 gene fusion or rearrangement (accelerated approval was granted previously in May 2021).
The planned phase 3 PROOF trial (NCT03773302) that was to test infigratinib versus gemcitabine plus cisplatin in the first line has been stopped due to poor enrollment.
Based on announcement, continued distribution of infigratinib for the second-line treatment of cholangiocarcinoma was deemed not to be commercially reasonable, so the sponsor voluntarily requested the withdrawal of infigratinib.
Despite this news, phase 2 proof-of-concept trial in patients with locally advanced or metastatic gastric cancer or gastroesophageal junction (GEJ) adenocarcinoma who are harboring FGFR2 gene amplification and who have failed at least 2 lines of previous standard therapy is ongoing (NCT06206278) with estimated completion next year.”
Additional information.
Source: Tanja Obradovic/LinkedIn
Tanja Obradovic is the Vice President of Oncology Scientific Affairs at ICON PLCh. She has over 20 years of clinical research experience and has led major pharmaceutical companies for 13 years. Her research focuses on small molecules, antibodies, cell and gene therapy, and major immunotherapy of PD1 inhibitors.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
