Ben Derman, Assistant Professor at the University of Chicago, shared on X/Twitter:
“Should MRD negativity be accepted as a surrogate endpoint for clinical trials in myeloma? What should be part of the FDA discussion tomorrow about MRD?
We need to first discuss the role of MRD as a prognostic biomarker. Perhaps the best visualization of this is a meta analysis by Nikhil Munshi that showed MRD – negative was associated with improved PFS and OS regardless of disease setting, sensitivity threshold, cytogenetic risk, IMWG response, or timing.
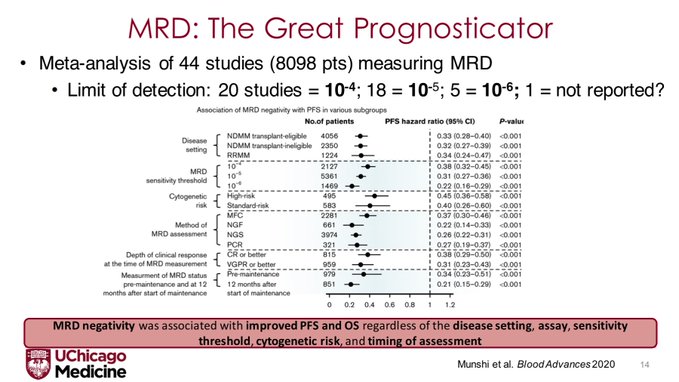
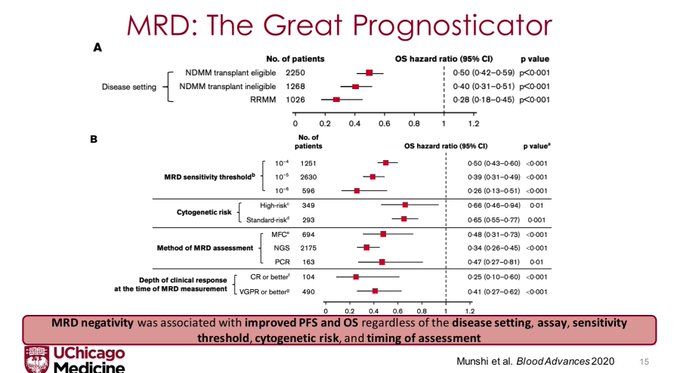
N.B. The deeper the MRD threshold, the better the prognosticative ability of the assay. This was also shown beautifully in the IFM-2009 data where each log fold increase in sensitivity was associated with improvements in PFS.
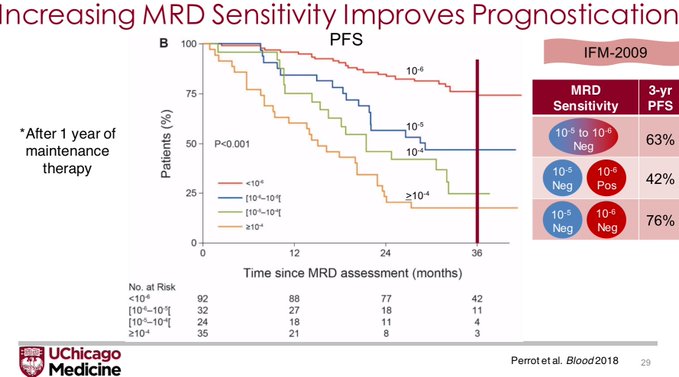
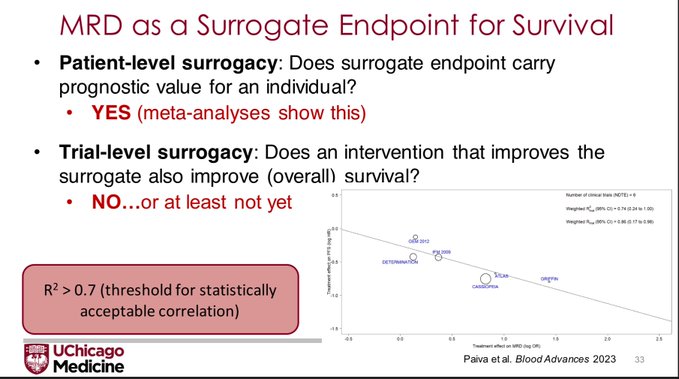
But that is showing that MRD has patient-level surrogacy. The FDA wants to see trial level surrogacy, that is, does an improvement in the MRD negativity rate lead to improvement in Overall Survival?
Bruno Paiva: When comparing randomized trials with a different in MRD negative of >12.3% vs <=12.3%, the median PFS between groups was not significant. Trials with Hazard Ratio <0.6 had larger difference in MRD(-). Correlation between MRD (-) and PFS showed R2=0.74 for NDMM (not bad).
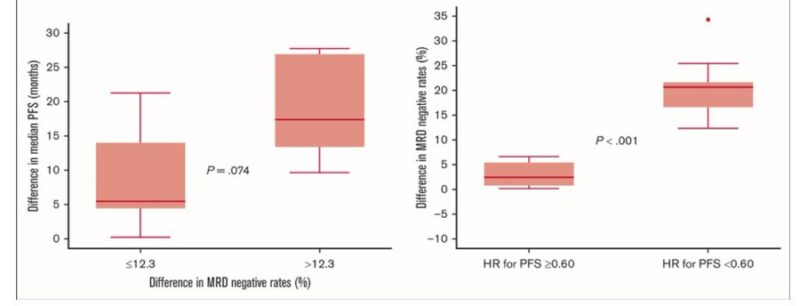
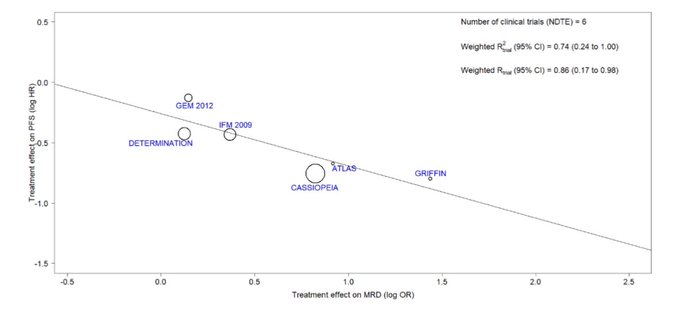
But this is looking at MRD (-) and PFS (not OS!). So trial level surrogacy is not proven by this analysis. And it will be tough to do so because of:
- Differences in MRD assays, sensitivity thresholds, and timing;
- Lack of OS difference between study arms in modern myeloma trials.
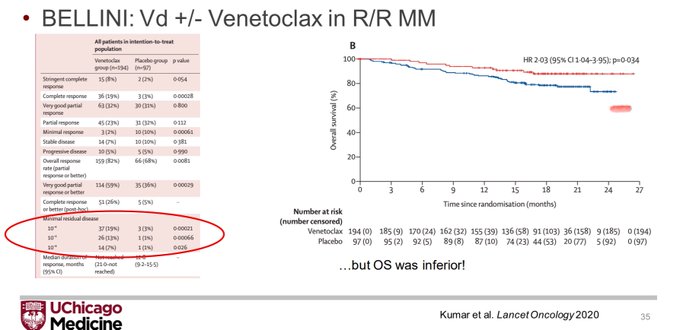
And why do we want to know about OS? The best example is the oft-referenced BELLINI trial where MRD negative was higher for venetoclax group but OS was worse.
In fact, if PFS is not a robust surrogate for OS in myeloma, then at best we are saying mrd neg is a surrogate of a so-so surrogate.
But…
- The FDA accepts conventional response as end point for traditional or accelerated pathways (see ide-cel, cilta-cel, bsab’s). That is far less impactful than MRD negative.
- An end point for accelerated approval will still require confirmation – but we get therapies to patients sooner.
- Many of the issues with MRD negativity still exist with PFS and yet most accept PFS as an end point.
- If we want to successfully employ more mrd adapted strategies, having an accepted endpoint can help to bolster this!
This is sure to ignite a lot of debate. Curious to hear your thoughts! Sound off below!”
Source: Ben Derman/X
About OncoDaily
OncoDaily was founded in 2023. It is a US-based oncology media platform, which features the latest news, insights, and patient stories from the world of oncology. Within a short period of time it became one of the leading oncology media platforms globally.
OncoDaily gathers content from various sources, including social media posts from renowned oncologists from all over the world, news from oncology societies and cancer centers, patient and survivor stories, and career-related information for professionals.
The mission of OncoDaily is to empower patients, survivors, and professionals with the knowledge and inspiration they need to fight cancer. The motto of OncoDaily is “Cancer doesn’t take a day off – neither do we”.
