
Toni Choueiri: The Renee Saliby kidney cancer biomarker paper in Cancer Cell is online with a detailed tweetorial
Toni Choueiri shared a post by Renee Maria Saliby, Postdoctoral GU Oncology Research Fellow at Dana-Farber Cancer Institute, on X:
“The Renee Maria Saliby kidney cancer biomarker paper in Cancer Cell is online with a detailed tweetorial: consistent benefit across all subgroups of IO-based therapies over VEGF TKI.”
Quoting Renee Maria Saliby’s post:
“Thrilled to share that our paper ‘Impact of RCC molecular subtypes on immunotherapy and targeted therapy outcomes’ is now published in Cancer Cell. Collaborative effort co-led with Chris Labaki and Tejas Jammihal under Eli Van Allen, Sachet Ashok Shukla, Toni Choueiri and David Braun‘s mentorship.
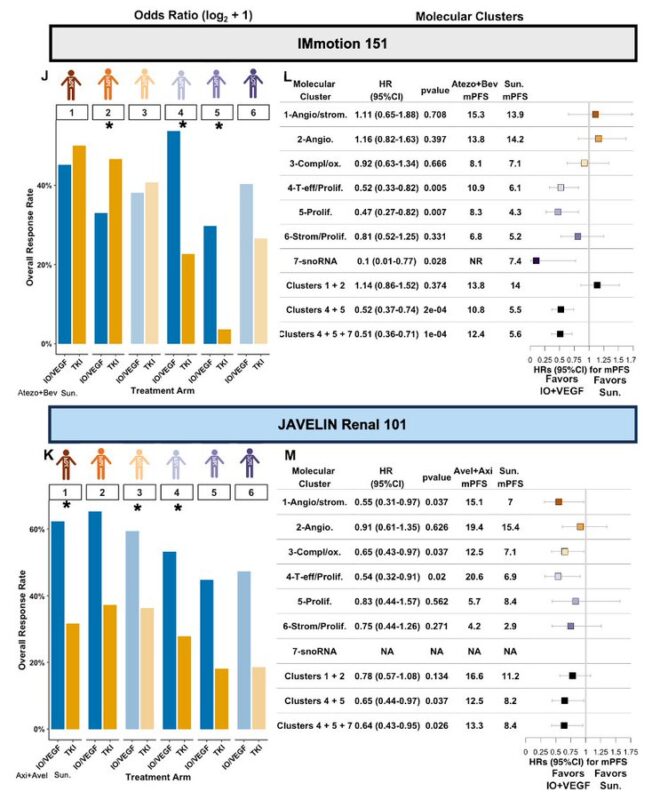
Despite great advances in the treatment landscape of Renal Cell Carcinoma, the field still lacks robust predictive biomarkers. The IM151 molecular subtypes were developed using NMF. Our aim was to evaluate the biology and predictive nature of these molecular clusters in the JR101 trial.
A machine-learning model assigned each patient to a molecular subtype according to RNA expression:
- angiogenic/stromal.
- angiogenic.
- complement/oxidation
- T-effector/proliferative.
- proliferative.
- stromal/proliferative.
- snoRNA.
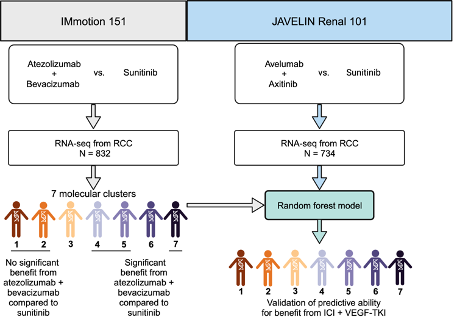
We performed an orthogonal analysis using bulk RNA-sequence, WES, baseline characteristics and clinical outcomes.
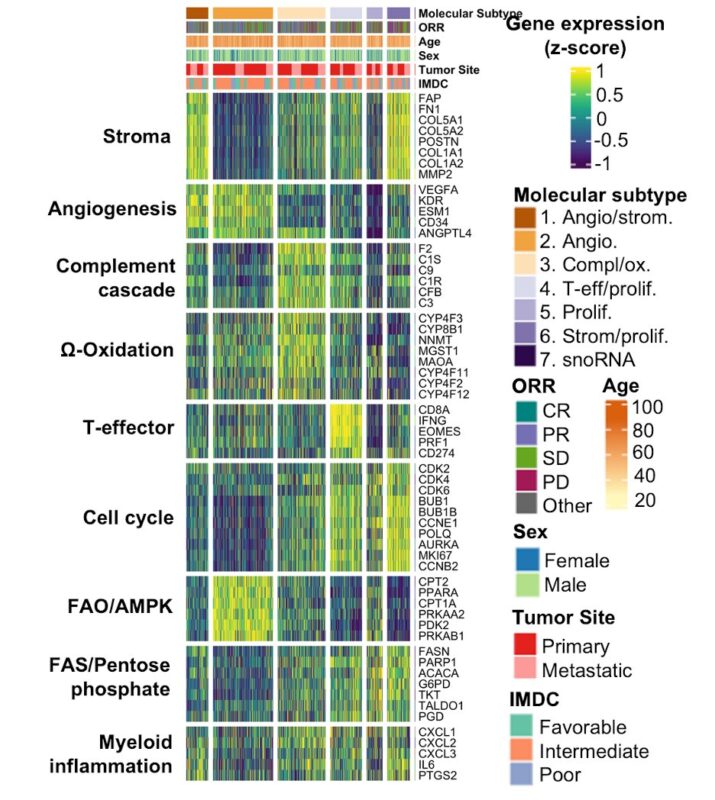
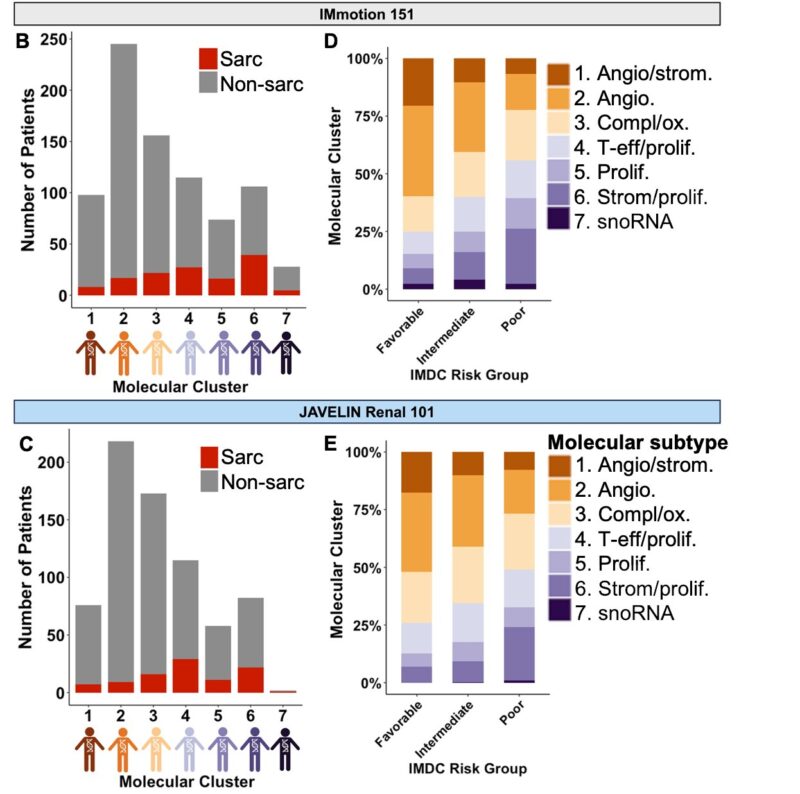
Read further.
Source: Toni Choueiri/X and Renee Maria Saliby/X
Toni K. Choueiri is the Director of the Lank Center for Genitourinary (GU) Oncology at Dana-Farber Cancer Institute (DFCI), co-leader of the Kidney Cancer Program at Dana-Farber/Harvard Cancer Center, and the Jerome and Nancy Kohlberg Chair and Professor of Medicine at Harvard Medical School. He is the Medical Director of International Strategic Initiatives at Dana-Farber and past President of the Medical Staff at DFCI (2016-2018). He received the George Canellos Award for Excellence in Clinical Investigation and Patient Care from DFCI in 2013, the Eugene Schonfeld Award from the Kidney Cancer Association (KCA) in 2016, and is a 2021 Giants of Cancer Care inductee. He serves on the National Comprehensive Cancer Network (NCCN) Kidney Cancer Panel, KidneyCan Board, the National Cancer Institute (NCI) GU Steering Committee, and is past Chairman (2015-2018) of the Medical and Scientific Steering Committee of the KCA. Dr. Choueiri is an elected member of the American Society of Clinical Investigation (ASCI). In addition, he is an Aresty Scholar from the Wharton School of Business at the University of Pennsylvania.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
