Photo from Paolo Tarantino/LinkedIn
Apr 8, 2024, 03:54
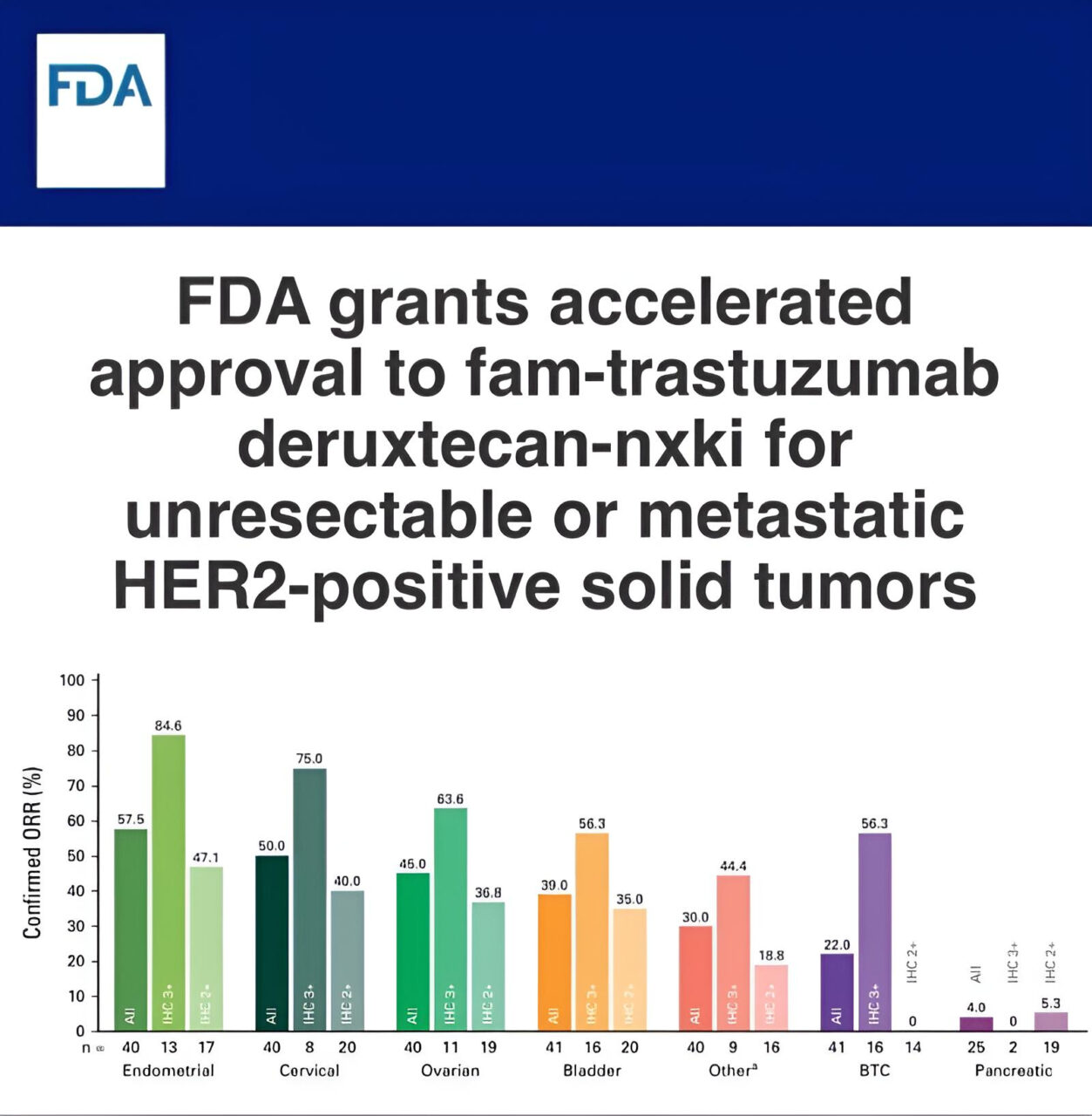
Paolo Tarantino: Trastuzumab deruxtecan is now FDA approved for ANY treatment-refractory HER2+ solid tumor
Paolo Tarantino, Advanced Research Fellow in the Breast Oncology Program at Dana-Farber Cancer Institute, shared a post on LinkedIn:
“Trastuzumab deruxtecan (T-DXd) is now FDA approved for the treatment of patients with ANY treatment-refractory HER2+ (IHC 3+) solid tumor, making it the first agnostic ADC. Unlikely to be the last. First priority: ensuring that HER2 testing is expanded across cancer types. Exciting times!”
Read further.
Source: Paolo Tarantino/LinkedIn
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
Aug 1, 2025, 19:24
Aug 1, 2025, 19:14
Aug 1, 2025, 18:32
