Jeffrey Martin, Founder of Oncoleader, recently shared on LinkedIn:
“Bispecific antibodies: what are they and who’s making them?
Okay, so, first things first.
A normal antibody is a small protein that is extremely good at one thing, recognizing and binding to another protein. Our body’s B cells naturally produce these things and, normally, they have an extremely high degree of specificity to ONE target. It’s kind of their thing…
Bispecific antibodies (bsAbs), on the other hand, are exactly what you’d expect them to be: antibodies that have a high degree of specificity to, not one, but two (or more) targets. It’s nice when scientists make it easy on us.
These have received much attention due to their anti-cancer potential and over the course of the last decade or so, many bsAbs have been developed for the treatment of various tumor types.
There are a vast array of different types of bsAbs that function in similar, but distinct ways. To understand the various mechanisms of action, you can think of them as being a part of these two broad categories:
1. Combinatorial
2. Obligate
– Combinatorial bsAbs are designed to kill two birds with one stone (inhibit two targets with one antibody). Many combinatorial bsAbs are designed to target two cancer-promoting entities, such as PD-L1 and CTLA-4. As one bsAb is binding to and inhibiting PD-L1, another is off somewhere else, binding to and inhibiting CTLA-4. It’s like smashing two different drugs into one molecule.
For example, check out Akesbio’s ‘Kaitanni’ PD1-CTLA4 bsAb or J& J’s MET-EGFR bsAb for NSCLC ‘Rybrevant’.
– Obligate bsAbs are meant to perform a function that could not be achieved by simply administering both antibodies separately. Many FDA-approved obligate bsAbs are referred to as ‘engagers’ (for example, bi-specific T cell engagers, or BiTEs).
These catalyze the engagement of particular immune cells – usually NK or T cells – to antigen-expressing tumor cells, enhancing the anti-tumor activity of the patient’s immune system. You can think of these as molecular magnets, drawing two separate entities together.
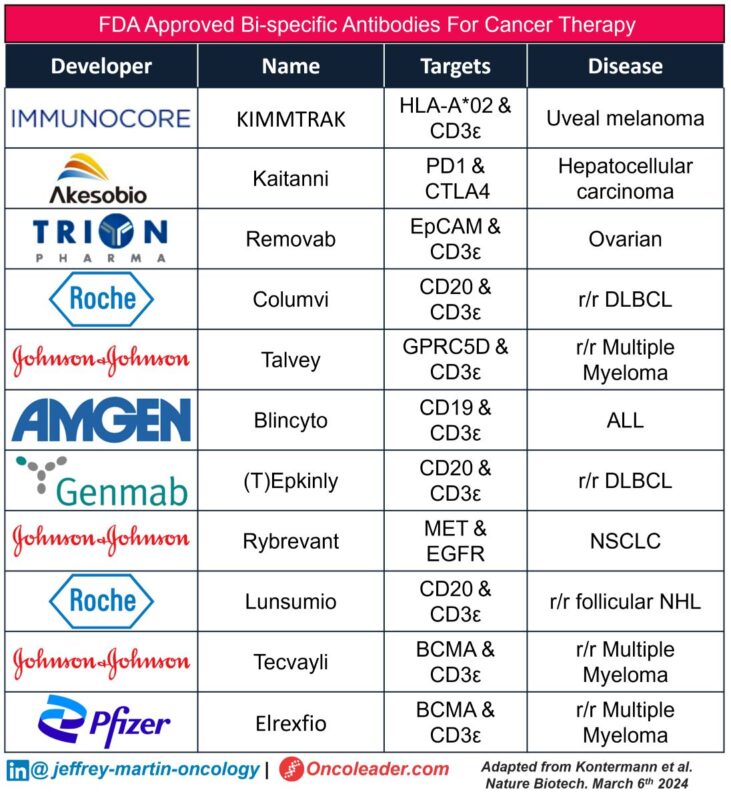
I’ve included a table of the 11 FDA-approved bsAbs for cancer therapy below.
The information in this table was obtained from a recent review paper published in Nature Reviews Drug Discovery. I will link the full article in the comments section.”
Source: Jeffrey Martin/LinkedIn
