Samer Al Hadidi, Assistant Professor at UAMS Myeloma Center, made the following post on X/Twitter:
“How to counsel patients about Cilta Cel using the data from CARTITUDE-4 and FDA ODAC meeting for earlier use.
With data of Cilta cel and Ide Cel, assuming having access to both for a given patient, I will personally choose Cilta cel (I explained this before).
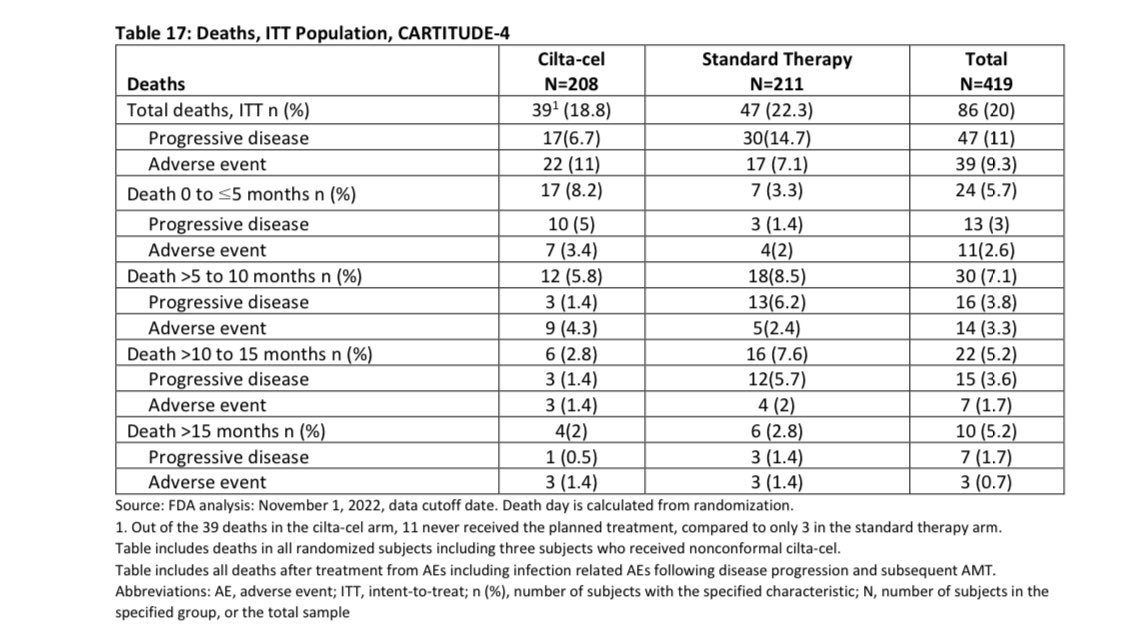
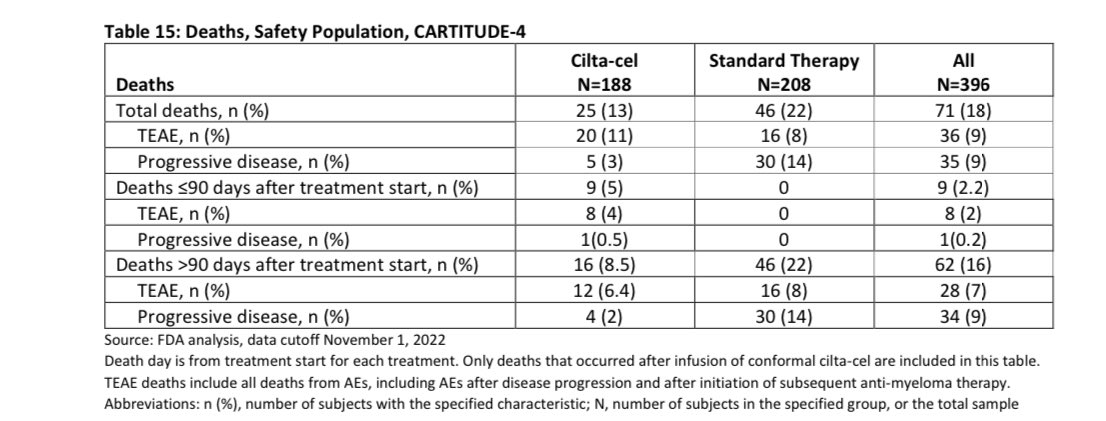
In the first ~6 months after we plan to give you Cilta-Cel, you have one out of 12 (8%) chances of dying either of progressive disease or adverse event to Cilta Cel, this will depend on the bridging therapy we will use and your response to it (planning is important to lower the risk).
If you are able to get your Cilta Cel, the risk of death in the first 3 months is 5% , most of those deaths (4%) were related to adverse events. Given the study was done in COVID era, some of the deaths were related to that infection so the risk maybe lower nowadays-espescially USA.
This risk of death is at least 4 times higher than autologous stem cell transplant which many already had in their treatment course , with improved supportive care and more experience this will be hopefully lower in the future.
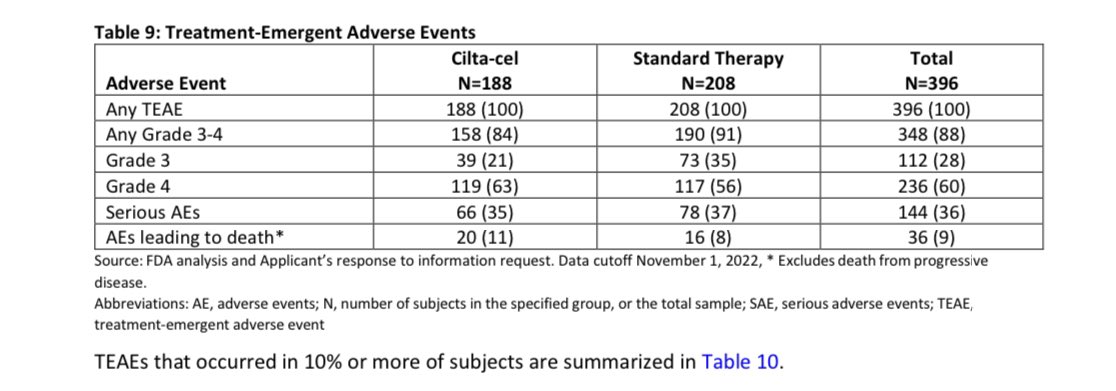
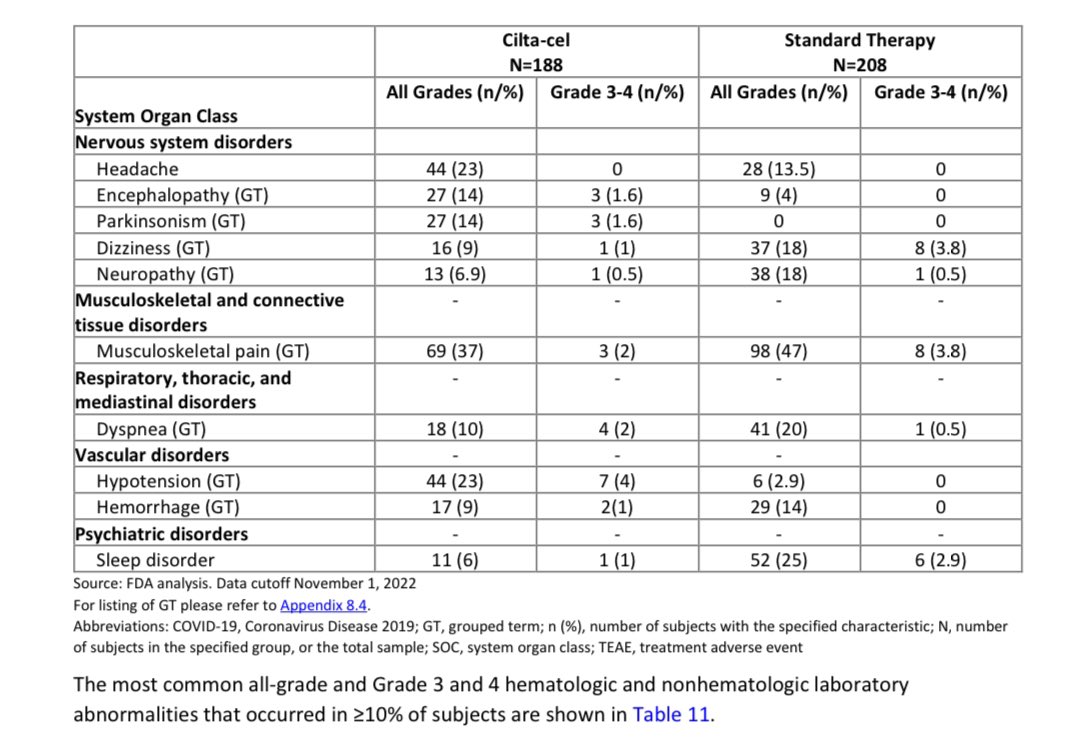
When you get Cilta-Cel, the more serious adverse event (grade 3 or 4) are common with one to 2 thirds of patients will have some , most resolve with time though it may require longer follow-up (months) and blood transfusions, infections, hospitalizations, need for stem cell support.
You have 3% chance of developing a secondary hematological cancer with this data being only accurate for an average of 1 year follow-up (15 months). We may know more in future if this is more or same. Those are hard to treat and shorten survival-risk higher with longer follow-up in initial trial.
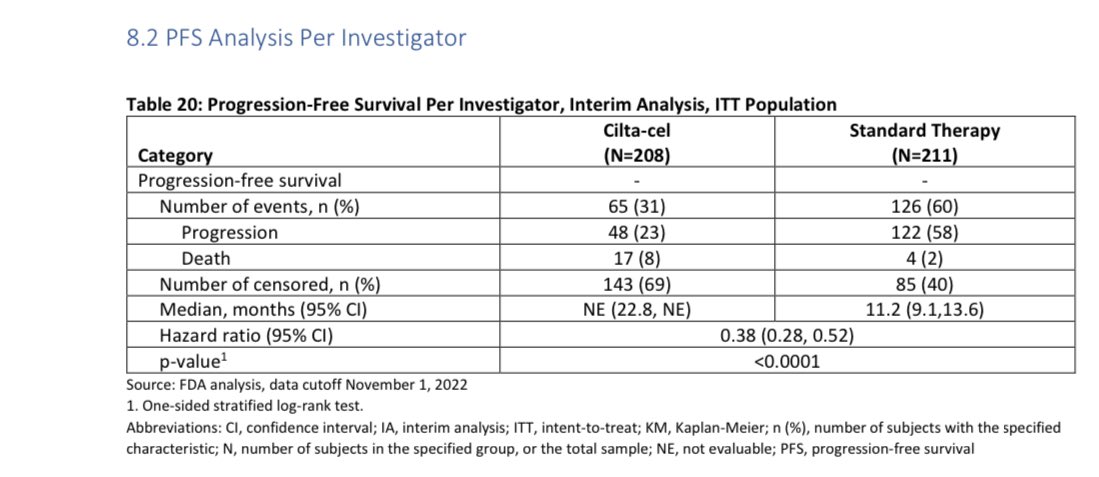
If you respond to Cilta Cel then you likely will respond for at least 2 years on average though some may respond for longer and we don’t have the average for the duration of response as of now- there will be treatment free interval with need to monitor your disease.
Your response duration will depend on many things including your disease characteristics, prior treatments and how good is disease control prior to infusion, but this response duration without need of ongoing therapy is a great plus for quality of life and many enjoy treatment free interval.
It is unclear if you will live longer if you receive Cilta Cel earlier than later, the study didn’t allow cross over, however earlier planning is key especially with triple class exposed/refractory.
We need longer follow up to see if this is true, though early data is promising.
There are neurological adverse events:
Short term neurological adverse events like facial palsy that usually resolve and more concerning Parkinson like syndrome (occurred in 14% of pts with <2% had higher grade)- we need longer follow up to assess long term effects/duration/outcomes.
If you are more than 75 years old, there is limited data on how you will do. In the trial <2% of patients were >75 years and the majority were <65 years old.
It is expected with older age that some side effects maybe more given more prevalent medical problems.
With expected future approval of Cilta cel in earlier lines,we need collaboration with community to avoid giving less effective therapies (ex: Selinexor, Elotuzumab , etc) and refer patients for discussion earlier to allow for planning. Not all options in NCCN menu are the same.
Such data argues against using this in smoldering myeloma.
While risk-benefit may favor Cilta Cel in relapsed and refractory myeloma, I will not put my asymptomatic patients with smoldering myeloma who may never progress into this risk.
Observation is the way to go and my recommendation.
A recent important paper on Blood Journal described characteristics of CAR-T related malignancies and this is ongoing to learn more about this risk.
Asymptomatic individuals should not take this risk, myeloma outcomes are only getting better.
We recommend observation for all patients in Arkansas regardless of risk and we enroll on our prospective trial with frequent testing according to risk.
An excellent trial by Manni Mohyuddin and Raj Chakraborty is investigating observation in high risk disease only.
Finally, we need to think globally. Myeloma patients outside of the US and even in many states in the US (vast majority of patients) don’t have access to Cilta Cel- this is extremely expensive procedure that we need to make more affordable with lowering cost and global use.”
Source: Samer Al Hadidi/X