10-Minute Immunotherapy Infusion: Safe, Efficient Delivery of Nivolumab and Pembrolizumab
Immunotherapy with immune checkpoint inhibitors (ICIs) such as nivolumab and pembrolizumab has revolutionized cancer treatment by targeting the PD-1/PD-L1 axis, resulting in durable responses across a broad spectrum of malignancies, including melanoma, non-small cell lung cancer, renal cell carcinoma, bladder cancer, head and neck squamous cell carcinoma, and gynecologic tumors. As the adoption of ICIs continues to grow with expanding indications and rising patient volumes, oncology clinics are increasingly challenged by limited infusion capacity and logistical constraints in care delivery.
Traditionally, nivolumab and pembrolizumab have been administered over 60 and 30 minutes, respectively, although these durations lack a pharmacologic basis. Emerging data now support a substantially shorter infusion time—specifically, 10-minute delivery—as a safe and efficient alternative that maintains therapeutic efficacy. This aligns with previous findings involving rapid infusion protocols for other monoclonal antibodies, including bevacizumab, rituximab, and trastuzumab.
This article examines the pharmacologic rationale, safety outcomes, and operational impact of 10-minute immunotherapy infusions, drawing on real-world evidence from a clinical implementation study. Broader implications for workflow efficiency, patient safety, and future directions within the evolving immunotherapy landscape are also discussed.
Pharmacologic Rationale for Accelerated Infusion
Both nivolumab and pembrolizumab possess long elimination half-lives of approximately 26 days, which ensures sustained systemic exposure across dosing intervals irrespective of infusion speed. As such, shortening the administration time does not alter the pharmacokinetics or reduce therapeutic efficacy.
The original rationale for prolonged infusion durations—60 minutes for nivolumab and 30 minutes for pembrolizumab—was based primarily on theoretical concerns about infusion-related hypersensitivity reactions (HSRs), rather than on pharmacologic or clinical necessity. However, emerging real-world data increasingly demonstrate that these ICIs can be administered safely at significantly faster rates without increasing the incidence or severity of HSRs. This challenges the need for conservative infusion schedules and opens the door to more efficient delivery models.
Implementation Strategy and Safety Monitoring
To evaluate the feasibility and safety of accelerated immunotherapy infusion, a clinical implementation project was conducted at Radboud University Medical Center (Radboudumc) in the Netherlands between January 2023 and December 2024. A total of 101 adult cancer patients receiving monotherapy with either nivolumab or pembrolizumab were enrolled, representing a variety of tumor types including melanoma, urological, gastrointestinal, head and neck, and gynecologic malignancies.
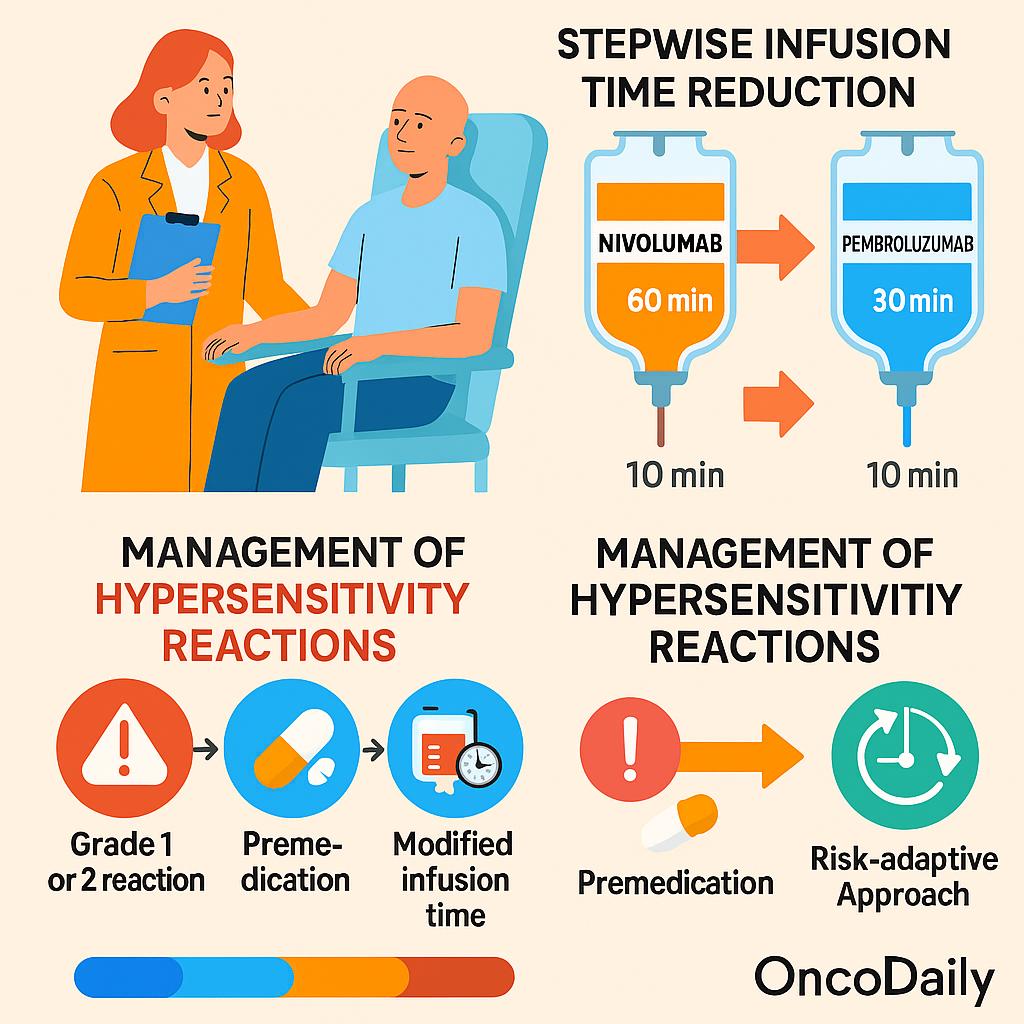
A structured, stepwise reduction protocol was adopted for infusion time adjustment. For nivolumab, the administration time was reduced from 60 minutes to 30 minutes and finally to 10 minutes over the course of 2–3 treatment cycles. For pembrolizumab, the schedule was simplified to two steps, transitioning directly from 30 minutes to 10 minutes.
To ensure patient safety, vital signs—including blood pressure, heart rate, and temperature—were monitored every 15 minutes starting from the initiation of infusion until 30 minutes after its completion during the initial treatment cycles. If no adverse events occurred, subsequent infusions were administered without extended monitoring.
Hypersensitivity reactions (HSRs) were managed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 and institutional protocols based on ESMO guidelines. Grade 1 reactions (mild, no intervention required) and grade 2 reactions (requiring temporary interruption and symptomatic treatment) were documented. In cases where a mild HSR occurred, future infusions were either administered at a longer duration or preceded by premedication with antihistamines or antipyretics. This risk-adaptive approach ensured that patients could safely continue treatment, often resuming accelerated infusion schedules once symptoms resolved.
Safety Outcomes and Hypersensitivity Events
Across 316 accelerated infusions administered during the implementation project, hypersensitivity reactions (HSRs) were observed in only 11 cases (3.5%), all of which were classified as grade 1 or 2. Importantly, no grade ≥3 HSRs occurred, supporting the overall safety of shortened infusion durations.
Notably, all reported HSRs occurred during nivolumab administration, with an incidence of 5% (11 out of 220 infusions), while no HSRs were recorded with pembrolizumab, underscoring its excellent tolerability—even at the 10-minute infusion rate. The rate of HSRs associated with nivolumab remained within the range reported in pivotal clinical trials and post-marketing data, suggesting that accelerated delivery does not elevate the risk beyond expected thresholds.
Interestingly, most reactions occurred after the second infusion, aligning with the possibility of anti-drug antibody (ADA) development contributing to immune sensitization. While these reactions were mild to moderate in severity, the management approach was tailored to each case. Several patients were able to continue treatment safely using an allergic schedule, premedication with antihistamines and antipyretics, or temporary reversion to a longer infusion time. In some cases, patients were successfully switched to pembrolizumab without recurrence of symptoms.
These findings demonstrate that with appropriate monitoring and management protocols, accelerated immunotherapy infusion is both safe and clinically manageable across diverse cancer populations.
Time Efficiency and Health System Impact
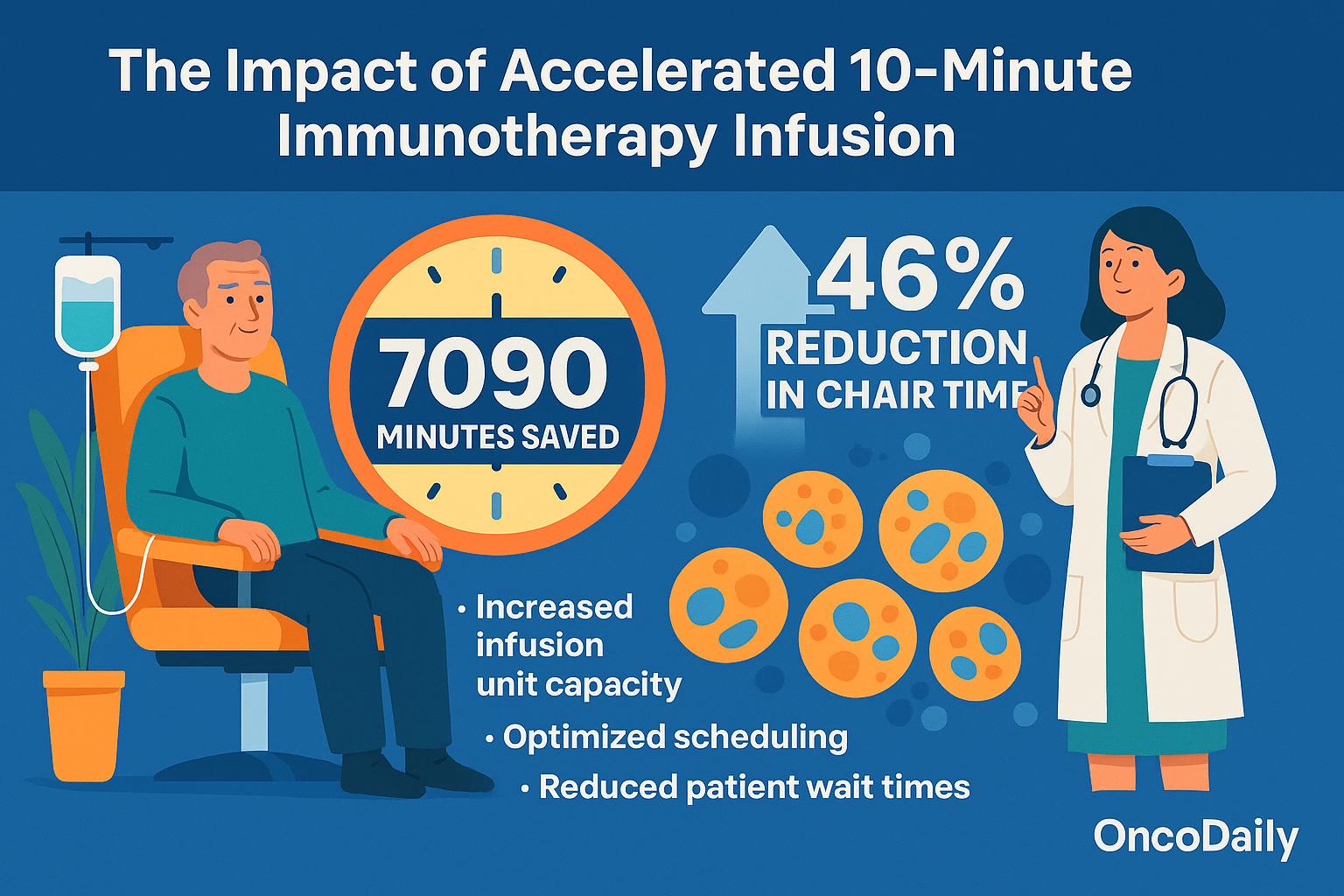
The implementation of accelerated 10-minute immunotherapy infusion yielded a substantial operational benefit, with a total of 7090 minutes (118 hours) saved across the study population. This represents a 46% reduction in chair timeover four treatment cycles when compared to standard infusion durations.
These time savings directly translate into increased infusion unit capacity, allowing more patients to be treated within the same clinical infrastructure—an increasingly critical advantage amid the rising global cancer burden and expanding indications for immune checkpoint inhibitors. By shortening the time per administration without compromising safety, oncology services can optimize scheduling, reduce patient wait times, and alleviate bottlenecks in high-volume infusion centers.
This streamlined approach contributes to a more sustainable model of cancer care delivery, where efficient resource utilization becomes as essential as therapeutic efficacy. As immunotherapy becomes standard across more tumor types and treatment lines, strategies like accelerated infusion will play a key role in maintaining accessibility and continuity of care in modern oncology practice.
Broader Implications and Clinical Scalability
The findings from this implementation study align closely with emerging trends in oncology toward more efficient and patient-friendly delivery methods, particularly the development of subcutaneous (s.c.) formulations of immunotherapy. The recent approval of s.c. atezolizumab in select regions reflects a growing interest in minimizing infusion-related resource use while maintaining efficacy and safety.
However, until s.c. options become widely accessible for all checkpoint inhibitors and indications, accelerated intravenous (IV) infusion offers a practical and scalable interim strategy. The demonstrated safety of 10-minute infusions for nivolumab and pembrolizumab supports this model as a complementary approach, particularly in institutions seeking to optimize capacity without compromising patient monitoring standards.
Given the shared pharmacokinetic characteristics among immune checkpoint inhibitors—such as long half-lives and low immunogenicity—this strategy may be extended to other monoclonal antibodies, including anti–PD-L1 (e.g., durvalumab, atezolizumab) and CTLA-4 agents (e.g., ipilimumab), provided a stepwise infusion reduction is validated in controlled settings.
While the potential for immediate 10-minute infusions is attractive from a logistical standpoint, current data support a gradual, stepwise reduction as the safer implementation model. This approach allows clinicians to monitor for hypersensitivity events during initial exposures and tailor future administrations accordingly. In routine practice, this measured transition ensures that operational efficiency does not come at the expense of patient safety—reinforcing a balanced, evidence-informed path toward more agile immunotherapy delivery.
Future Directions and Recommendations
To strengthen the evidence base and support broader adoption of accelerated immunotherapy infusion, multi-center prospective studies are warranted. Such trials should include diverse patient populations, tumor types, and treatment settings to validate the safety and efficacy of 10-minute infusion protocols on a larger scale.
In parallel, cost-effectiveness analyses are essential—particularly in resource-constrained environments, where optimizing infusion capacity can have a significant impact on accessibility and continuity of care. Demonstrating financial and operational benefits will further support the case for integration into routine oncology practice.
As real-world evidence continues to accumulate, there is a growing need for regulatory bodies to revisit current infusion time guidelines. Labeling revisions that reflect updated clinical experience with rapid infusion protocols would provide clarity and flexibility for clinicians, aligning product use with evolving standards of care.
Finally, the development of evidence-based clinical guidelines is a critical next step. These should outline standardized, stepwise protocols for infusion time reduction, premedication strategies, and monitoring recommendations, ensuring consistency across institutions while preserving individualization based on patient risk. Such guidelines will facilitate safe and systematic implementation of time-efficient immunotherapy across global oncology networks.
You Can Read The Article here
You Can Watch More on OncoDaily Youtube TV
FAQ
What is a 10-minute immunotherapy infusion?
A rapid delivery method of ICIs like nivolumab/pembrolizumab, reducing infusion time to 10 minutes safely.
Is 10-minute infusion safe?
Yes—studies show low rates of mild hypersensitivity reactions with proper monitoring.
Which drugs are used in accelerated infusion?
Nivolumab and pembrolizumab are the focus; others may follow.
How is patient safety ensured during rapid infusion?
Vital signs are monitored during early infusions; protocols guide management of mild reactions.
What cancers are treated using this method?
Melanoma, lung, renal, bladder, HNSCC, and gynecologic cancers, among others.
How much time does it save?
About 7090 minutes (118 hours) saved across 316 infusions—roughly 46% reduction in chair time.
What are common side effects?
Only mild (grade 1–2) hypersensitivity reactions were observed—no severe events.
Can this apply to other ICIs like durvalumab?
Potentially, yes—pending validation in similar stepwise protocols.
How does this help oncology clinics?
Improves infusion capacity, reduces wait times, and supports care scalability.
Will this replace subcutaneous immunotherapy?
No—it complements it until s.c. options become widely available.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023