Lonsurf (Trifluridine/Tipiracil): A Patient’s Guide 2025 to Treatment for Advanced Colorectal and Stomach Cancer
Lonsurf is a cancer medicine taken by mouth to treat some types of advanced digestive system cancers, specifically metastatic colorectal cancer and gastric (stomach) cancer. It combines two drugs—trifluridine and tipiracil—to attack cancer cells. The U.S. Food and Drug Administration (FDA) first approved Lonsurf in 2015 for patients with colorectal cancer that no longer responds to standard treatments. Since then, it has also been approved for advanced stomach cancer and, more recently, in combination with another drug, bevacizumab, for colorectal cancer.
Lonsurf offers hope for patients who have few other options left, helping to control cancer growth and improve survival while allowing many patients to continue treatment at home.
What Is Lonsurf and How Does It Work?
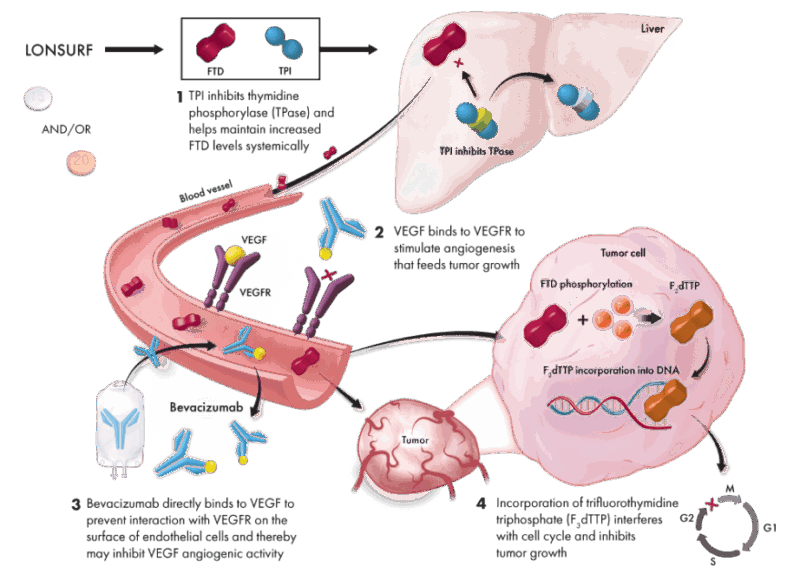
Lonsurf is an oral chemotherapy made up of two active ingredients. The first, trifluridine, works by getting inside cancer cells and interfering with their DNA. Specifically, it inserts itself directly into the DNA of cancer cells during their division process, which damages the DNA and prevents the cancer cells from multiplying. This effect ultimately causes cancer cell death.
However, trifluridine on its own is quickly broken down and inactivated by the body. This is where the second component, tipiracil, comes in. Tipiracil blocks an enzyme called thymidine phosphorylase, which normally breaks down trifluridine. By inhibiting this enzyme, tipiracil helps trifluridine stay in the bloodstream longer, increasing its concentration and allowing it to be more effective in reaching and killing cancer cells.
Together, this combination ensures that trifluridine remains at an active level in the body long enough to disrupt cancer cell DNA and fight tumors, especially those that have become resistant to other chemotherapy drugs like 5-fluorouracil. After taking Lonsurf, the half-life—the time it takes for half of the drug to leave your body—is approximately 2.1 hours for trifluridine and 2.4 hours for tipiracil. This relatively short half-life means the drug cycles through the body fairly quickly, which is why it is taken twice daily on a specific schedule.
What Cancers Does Lonsurf Treat?
Lonsurf is mainly used to treat cancers of the digestive system that have spread to other parts of the body (metastatic cancers) and that no longer respond to standard treatments. The main cancers approved for Lonsurf treatment are:
- Metastatic colorectal cancer (mCRC): Lonsurf is used in patients who have already been treated with standard chemotherapy drugs such as fluoropyrimidines (like 5-FU), oxaliplatin, irinotecan, anti-angiogenic therapies (such as bevacizumab), and for those with specific genetic features (RAS wild-type), anti-EGFR agents.
- Advanced gastric (stomach) cancer and gastroesophageal junction cancer: Lonsurf is approved for patients who have received at least two previous chemotherapy treatments without success.
- Metastatic colorectal cancer combined with bevacizumab: In 2023, the FDA approved Lonsurf together with bevacizumab for patients with previously treated mCRC, offering improved outcomes compared to Lonsurf alone.

You can read about Gastric Cancer and Smoking — What Are the Shocking Real Risks? on OncoDaily.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Lonsurf was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
Clinical trials have carefully studied how well Lonsurf works for people with advanced colorectal and stomach cancers. These studies show that Lonsurf can help control the cancer, extend life, and improve daily functioning for patients who have already undergone other treatments. Here’s a closer look at the main trial results.
Lonsurf for Metastatic Colorectal Cancer
The approval of Lonsurf for metastatic colorectal cancer was based on a large clinical study called the RECOURSE trial, which included 800 patients whose cancer continued to grow despite multiple prior treatments. In this study, patients who took Lonsurf lived longer on average compared to those who received a placebo (a dummy pill).
Specifically, the median overall survival (the time by which half of the patients are still alive) was 7.1 months for patients treated with Lonsurf versus 5.3 months for those who received placebo. This means Lonsurf extended life by nearly two months on average. Patients taking Lonsurf also experienced a longer time before their cancer worsened, known as progression-free survival, and maintained better daily functioning for longer periods. Side effects were manageable, with the most common being low white blood cells, which can increase infection risk, anemia, nausea, and fatigue.
Lonsurf for Advanced Gastric and Gastroesophageal Junction Cancer
In patients with stomach or gastroesophageal junction cancer, a large international study known as the TAGS trial examined Lonsurf’s effectiveness. This study included patients who had already undergone at least two prior chemotherapy treatments.
The results showed that patients treated with Lonsurf lived on average 4.8 months, compared to 3.5 months for those who received supportive care without active chemotherapy. While this might seem like a small difference, it was statistically significant and meaningful for patients with few other options. The drug also delayed disease progression and maintained quality of life. Common side effects were similar to those seen in colorectal cancer patients, including low blood counts and gastrointestinal symptoms.
Lonsurf Combined with Bevacizumab for Colorectal Cancer
The SUNLIGHT trial, published in 2023, tested Lonsurf together with bevacizumab (a drug that blocks blood vessel growth to tumors) in patients with metastatic colorectal cancer who had been previously treated. This combination improved survival more than Lonsurf alone. Patients lived a median of 10.8 months compared to 7.5 months with Lonsurf alone. The time patients lived without their cancer getting worse was also longer (5.6 months vs. 2.4 months). This combination also helped patients maintain their daily activities for longer, delaying decline in physical function.

You can also read about Colon Cancer Cure Rate: What Patients Should Know in 2025 on OncoDaily.
Lonsurf Side Effects and Management
Lonsurf can cause several side effects, mainly because it affects not only cancer cells but also healthy cells that grow quickly, such as those in your bone marrow and digestive tract. The most common side effect is a low number of white blood cells, especially neutrophils, which are important to fight infections. This condition, called neutropenia, can increase your risk of infections and may require treatment adjustments or medications to stimulate white blood cell production.
Fatigue is also common, making patients feel tired or weak. Low red blood cells (anemia) can cause shortness of breath and tiredness, while low platelets (thrombocytopenia) can increase bruising or bleeding risk. Gastrointestinal issues like nausea, vomiting, diarrhea, loss of appetite, and stomach discomfort may also occur.
Less Common Side Effects
Less frequently, patients might experience changes in taste, dry mouth, mouth sores, mild skin rashes or itching, hair thinning, headaches, or dizziness. While these are less common, they can affect your comfort and should be discussed with your healthcare team.
Managing Side Effects
Managing side effects early is essential for staying on treatment and maintaining quality of life. Your healthcare team will monitor your blood counts closely. If your white blood cells drop too low, your doctor may pause treatment or reduce the dose. They might also prescribe medications called growth factors to help your body produce more white blood cells. For nausea and vomiting, anti-nausea medications can be used before or during treatment. Diarrhea may be controlled with dietary changes and medicines like loperamide. Rest, hydration, and light exercise can help reduce fatigue.
Good oral care, such as gentle brushing and rinses, can ease mouth sores or dryness. Skin reactions may be treated with creams or antihistamines if needed. Regular communication with your healthcare team helps catch problems early, so don’t hesitate to report new or worsening symptoms.

What Is the Recommended Dosage of Lonsurf?
The dose is based on your body surface area, usually 35 mg/m² twice daily on days 1 to 5 and days 8 to 12 in a 28-day cycle. The maximum single dose should not exceed 80 mg. For patients who experience significant side effects, doctors may reduce the dose in steps, but the dose should not be increased once lowered. In combination with bevacizumab for colorectal cancer, bevacizumab is given by IV at 5 mg per kilogram of body weight on days 1 and 15 of each cycle. It is crucial to follow your doctor’s dosing instructions carefully and report any side effects.
How Is Lonsurf Administered?
Lonsurf comes as oral tablets that you take with food to improve absorption and reduce stomach upset. Swallow the tablets whole without crushing or chewing. You should not try to make up missed doses but continue your normal schedule. Store the medication at room temperature, and if you remove tablets from the original bottle, use them within 30 days. Because Lonsurf is a chemotherapy drug, it requires careful handling—wash your hands after touching the tablets and follow disposal instructions.
What Can You Expect During Treatment?
Lonsurf is taken by mouth as tablets, usually twice a day for five days in a row, followed by two days off, then another five days on, completing a 28-day cycle. Your doctor will calculate the right dose based on your body size and health status, but a common dosing schedule is 35 mg/m² twice daily on days 1–5 and 8–12. If you are also receiving bevacizumab, it will be given as an intravenous (IV) infusion in the clinic on days 1 and 15.
Before each cycle of treatment, your healthcare team will perform blood tests to check your blood cell counts and make sure it is safe to continue. If side effects like low blood cells are severe, treatment may be delayed or doses adjusted to keep you safe.
During each dose, you should take Lonsurf with food, swallowing the tablets whole without crushing or chewing them. It is important not to skip doses, and if you miss one, do not double the next dose but continue with your regular schedule. Treatment cycles repeat every 28 days and usually continue until your doctor determines that the treatment is no longer controlling the cancer or if side effects become too severe.
Lonsurf’s Metabolism and Elimination
Unlike many drugs, Lonsurf is not processed by the liver’s cytochrome P450 system, which lowers the chance of interactions with other medicines you may be taking. Trifluridine, the active chemotherapy agent, is broken down mainly by thymidine phosphorylase into an inactive substance called 5-(trifluoromethyl) uracil (FTY). The half-life, or the time it takes for half the drug to leave your body, is about 2.1 hours for trifluridine and 2.4 hours for tipiracil, so the drug cycles quickly through your system, which is why regular dosing is important.
What Should You Avoid During Lonsurf Treatment?
It is important not to miss doses or double doses. Always take Lonsurf with food and swallow tablets whole. Avoid starting any new medications, including over-the-counter drugs, supplements, or herbal remedies, without your doctor’s approval to prevent unwanted interactions. Alcohol intake should be discussed with your healthcare provider, as it might worsen side effects or interfere with treatment. Live vaccines should be avoided during treatment due to weakened immune response.
Women who are pregnant or planning to become pregnant should not take Lonsurf, as it can harm the unborn baby. Effective contraception is essential during treatment and for at least six months afterward. Breastfeeding should be stopped during treatment and for one day after the last dose. Monitor closely for any signs of infection, unusual bleeding, or other symptoms, and report them promptly.
Real-Life Effectiveness
A large real-world study published in the Journal of Clinical Oncology in May 2025 examined patients treated with Lonsurf with or without bevacizumab in everyday clinical practice. The study included 265 patients mostly receiving third-line treatment for metastatic colorectal cancer.
Those treated with Lonsurf plus bevacizumab lived a median of 11.6 months, almost twice as long as those treated with Lonsurf alone, who lived about 6.2 months. The time before needing another treatment or death was also longer with the combination (9.4 months vs. 5.8 months). The safety profile was similar to clinical trials, though low white blood cells occurred more often in the combination group. These real-world results support the benefits of adding bevacizumab to Lonsurf in improving survival and quality of life.
What Other Trials Are Ongoing?
Scientists are continuing to study Lonsurf in new ways. One ongoing clinical trial, called REGTAS-2 (NCT06992648), is comparing Lonsurf combined with regorafenib (another cancer drug) versus Lonsurf with bevacizumab in patients with metastatic colorectal cancer. The goal is to find out which combination works better or if they are equally effective.
In addition to this, researchers are exploring whether Lonsurf can be used in other types of cancers or combined with new therapies, potentially offering more treatment options in the future.
If you’re a healthcare provider, access the professional version here.
Written by Mariam Khachatryan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
