Day 1 of GU24 ASCO Highlights by Oncology Brothers
Oncology Brothers shared on their X/Twitter page:
“Day 1 GU24 ASCO Highlights
1. CONTAC02: Atezo/Cabo mCRPC
2. BRCAAWAY: PARPi in mCRPC
3. EMBARK: High-Risk biochemical recurrence prostate cancer

1. CONTACT02: After 1 prior novel hormonal Rx (NHT), Cabo + Atezo vs 2nd NHT
– Cabo/Atezo with high PFS (6.3 mos vs 4.2mos, HR: 0.65) and trend towards OS (16.7mos vs 14.6mos, HR: 0.79)
– 2nd NHT often has poor outcomes. – Cabo alone has some activity (COMET studies), unclear how much is Atezo adding.
– Need more long term data.
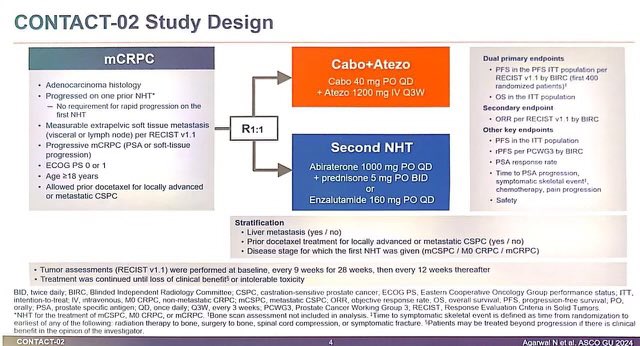
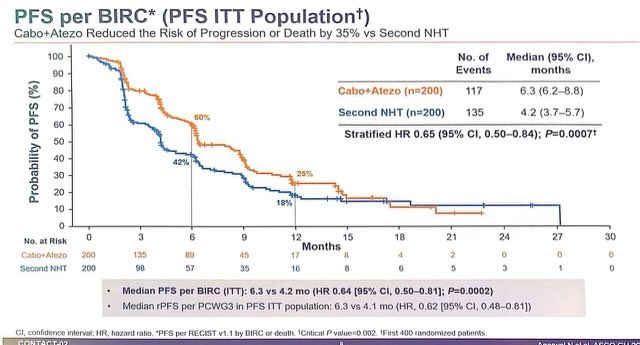
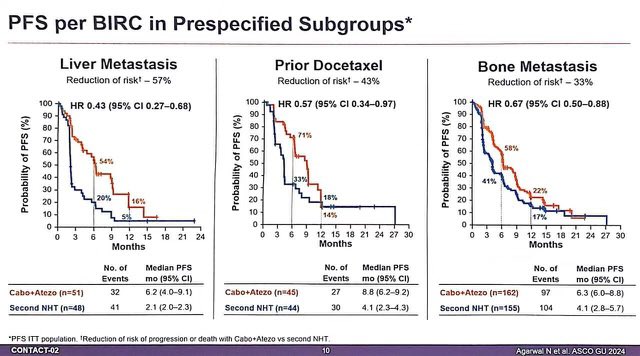

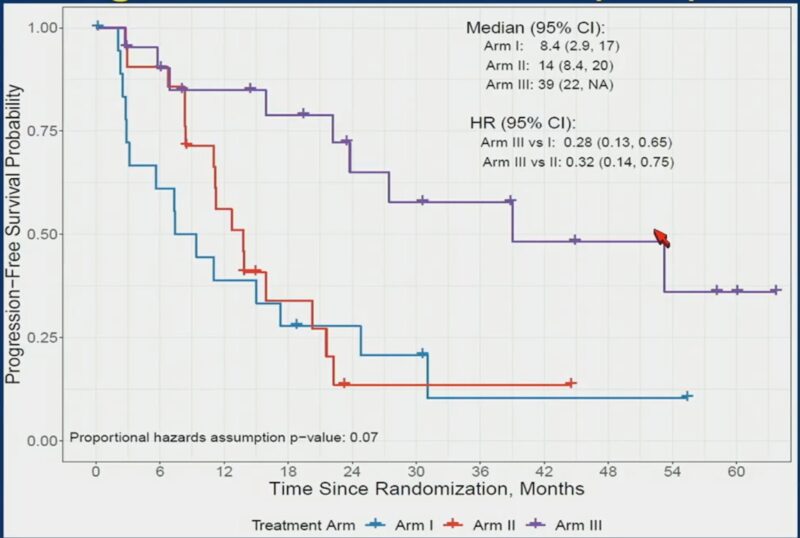
2. BRCAAWAY: Ph II, n=165, mCRPC with BRCA 1/2 or ATM mutation, Abi vs Olaparib (sequential/crossover allowed) vs Abi/Olaparib:
– Significant high PFS with combo 39mos vs 8.4mos with Abi vs 14mos with Olaparib (PFS2 could be an important)
– Combo (already approved) should be considered/SoC for mCRPC with BRCA 1/2 and ATM.
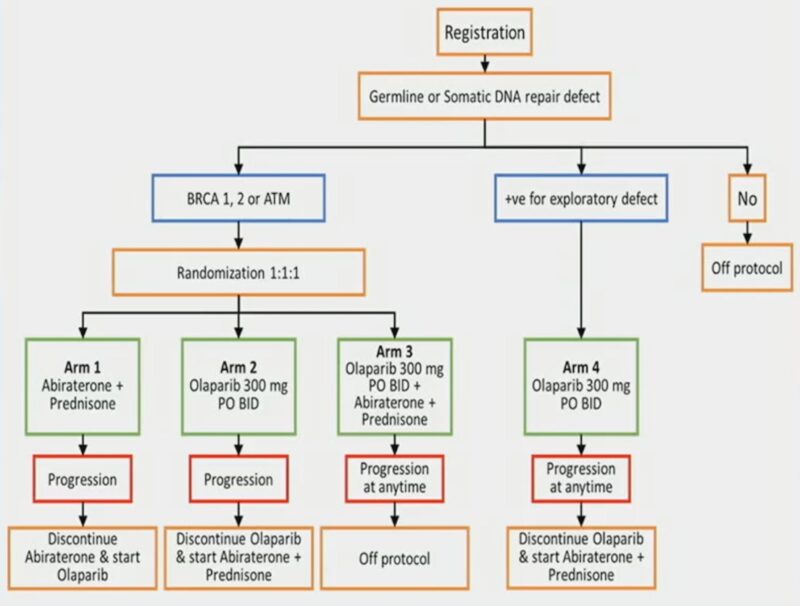


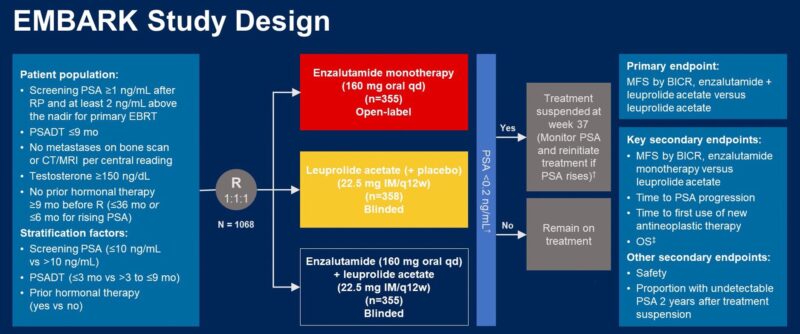
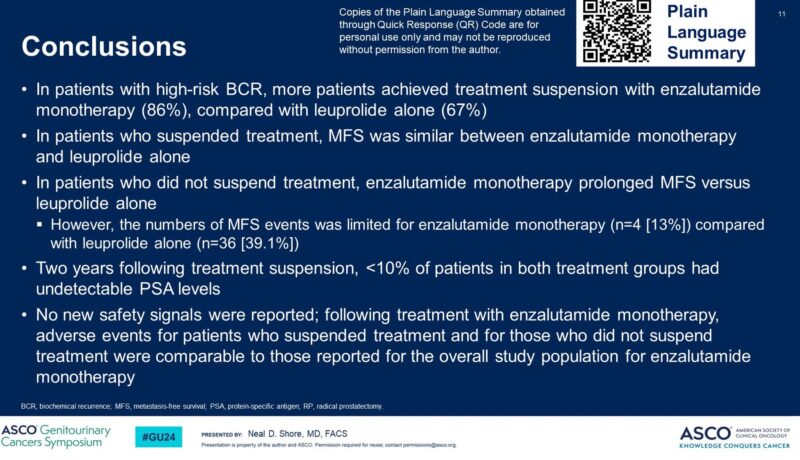
3. EMBARK: Enzalutamide was approved in November 2023 for non-metastatic CSPC with biochemical relapse based on this study.
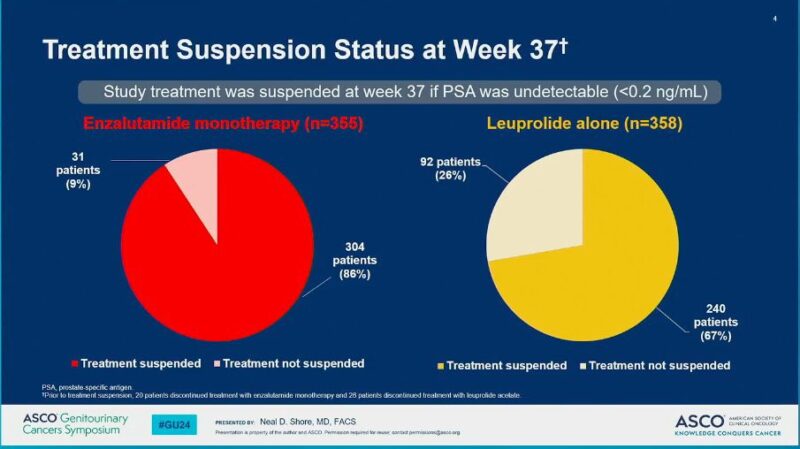
– Monotherapy with Enza could be a preferred option in some (higher number of patients were able to achieve undetectable PSA and stop Rx in comparison to Lupron)
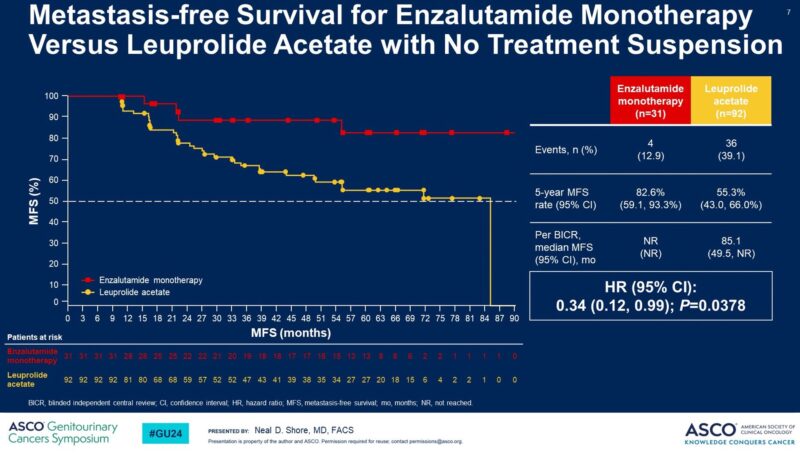
– Improved MFS with Enza monotherapy when Rx not suspended.”
Source: Oncology Brothers/X
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
