BEATcc Trial at ESMOGynaec25: Atezolizumab Benefits Cervical Cancer Patients Regardless of PD-L1 Status
The BEATcc Phase III trial took center stage at ESMO Gynaecology 2025, as Dr. Kristina Lindemann of Oslo, Norway, presented new data exploring the impact of PD-L1 status on outcomes in patients with recurrent, persistent, or metastatic cervical cancer treated with atezolizumab, chemotherapy, and bevacizumab.
Background
Recurrent, metastatic, or persistent cervical cancer (R/M CC) remains a significant clinical challenge with poor long-term outcomes. While the addition of bevacizumab to platinum-based chemotherapy has modestly extended survival, durable responses remain rare, and most patients ultimately experience disease progression. In this setting, immune checkpoint inhibitors—particularly anti–PD-L1 therapies—have emerged as promising treatment options, but their optimal use and patient selection strategies are still evolving.
The BEATcc Phase III trial (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030) previously demonstrated that incorporating atezolizumab (atezo), a PD-L1 inhibitor, into first-line treatment with chemotherapy (CT) and bevacizumab (bev) led to statistically significant improvements in both progression-free survival (PFS) and overall survival (OS) compared to CT + bev alone. Notably, median OS exceeded 2.5 years, marking a meaningful advancement in the management of advanced cervical cancer.
However, a critical unanswered question is whether PD-L1 expression, commonly assessed via the combined positive score (CPS), serves as a predictive biomarker in this context. While PD-L1 status has been used to guide immunotherapy in other tumor types, its utility in R/M CC remains unclear. The post hoc analysis presented at ESMO Gynaecology 2025 aimed to clarify this by examining the impact of PD-L1 status on survival outcomes within the BEATcc trial, helping to inform whether treatment decisions should be stratified based on biomarker expression.
Methods
The BEATcc trial (NCT03556839; ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030) is a global, open-label, randomized phase III study designed to evaluate the efficacy and safety of adding atezolizumab, an anti–PD-L1 immune checkpoint inhibitor, to standard chemotherapy (paclitaxel + cisplatin or carboplatin) and bevacizumab in patients with previously untreated recurrent, persistent, or metastatic cervical cancer (R/M CC).
Eligible patients were adults with measurable R/M CC not amenable to curative surgery or radiotherapy, and with no prior systemic treatment in the advanced setting. Patients were randomized in a 1:1 ratio to receive:
- Arm A (Experimental): Atezolizumab (1200 mg IV, day 1 every 3 weeks) + chemotherapy + bevacizumab
- Arm B (Control): Chemotherapy + bevacizumab
Treatment continued until disease progression or unacceptable toxicity. Stratification factors included histology (squamous vs. non-squamous), geographic region, and choice of platinum agent (cisplatin vs. carboplatin).
The dual primary endpoints were:
- Progression-free survival (PFS)
- Overall survival (OS)
Key secondary endpoints included:
- PFS2 (time from randomization to progression on next-line therapy or death)
- Objective response rate (ORR)
- Duration of response (DoR)
- Safety and tolerability
Results
In this post hoc biomarker analysis of the BEATcc trial, outcomes were evaluated according to PD-L1 expression using CPS thresholds of 1 and 10. Among the 313 patients in the biomarker-evaluable population, survival benefits with the addition of atezolizumab were observed across all PD-L1 subgroups, including those with CPS <1, indicating that PD-L1 expression did not significantly alter the efficacy of the treatment combination.
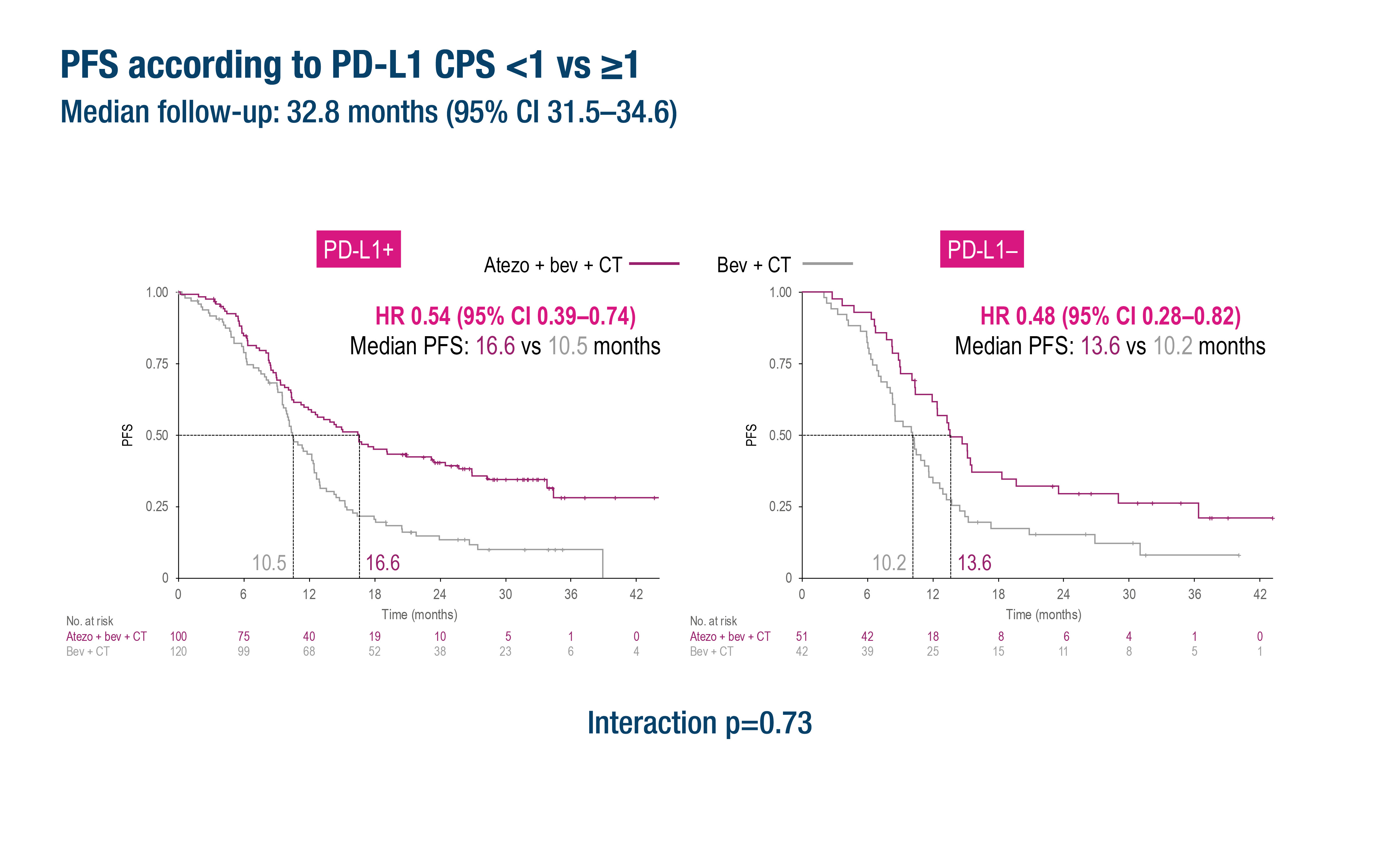
CPS <1 (n=93):
- PFS HR = 0.48 (95% CI: 0.28–0.82)
- OS HR = 0.43 (95% CI: 0.24–0.77)
CPS ≥1 (n=220):
- PFS HR = 0.54 (95% CI: 0.39–0.74)
- OS HR = 0.73 (95% CI: 0.51–1.06)
CPS ≥10 (n=101):
- PFS HR = 0.54 (95% CI: 0.32–0.91)
- OS HR = 0.65 (95% CI: 0.34–1.23)
Notably, interaction tests for PD-L1 status were not statistically significant for PFS (p=0.73), PFS2 (p=0.53), or OS (p=0.12), indicating no predictive effect of PD-L1 expression on treatment efficacy.
Key Takeaways
- Atezolizumab provided clinical benefit across all PD-L1 subgroups, including PD-L1-negative (CPS <1) patients.
- PD-L1 is not a reliable biomarker for selecting patients for immunotherapy in R/M cervical cancer.
- The median OS with atezo + CT + bev exceeded 2.5 years, marking a substantial advancement in the treatment of this population.
You can read the full abstract here.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
