
SOHO-01 Trial Results: BAY 2927088 Shows Promising Activity in Advanced HER2-Mutant NSCLC at ASCO 2025
BAY 2927088 is a potent, reversible HER2 tyrosine kinase inhibitor that has demonstrated manageable safety and anti-tumor activity in patients with advanced non-small cell lung cancer (NSCLC) harboring HER2-activating mutations. The SOHO-01 trial is an ongoing, open-label, multicenter Phase I/II study designed to further evaluate the safety and efficacy of BAY 2927088 in this patient population.
Study Design and Patients
Eligible patients with advanced NSCLC and HER2-activating mutations received oral BAY 2927088 at 20 mg twice daily.
- Cohort D: Patients with disease progression after ≥1 systemic therapies, naïve to HER2-targeted therapy.
- Cohort F: Patients with no prior systemic therapy for locally advanced or metastatic disease.
The primary endpoint was safety (MedDRA v27.1 and CTCAE v5.0). Anti-tumor activity (RECIST v1.1) was a key secondary endpoint.
Key Results
As of October 14, 2024, 81 patients in Cohort D and 39 in Cohort F were treated. Median ages were 60 and 65 years, respectively. Females comprised 61.7% (D) and 64.1% (F); never-smokers were 61.7% (D) and 79.5% (F); and 43.2% (D) had received ≥2 prior systemic therapies. All patients were included in the safety and efficacy analyses.
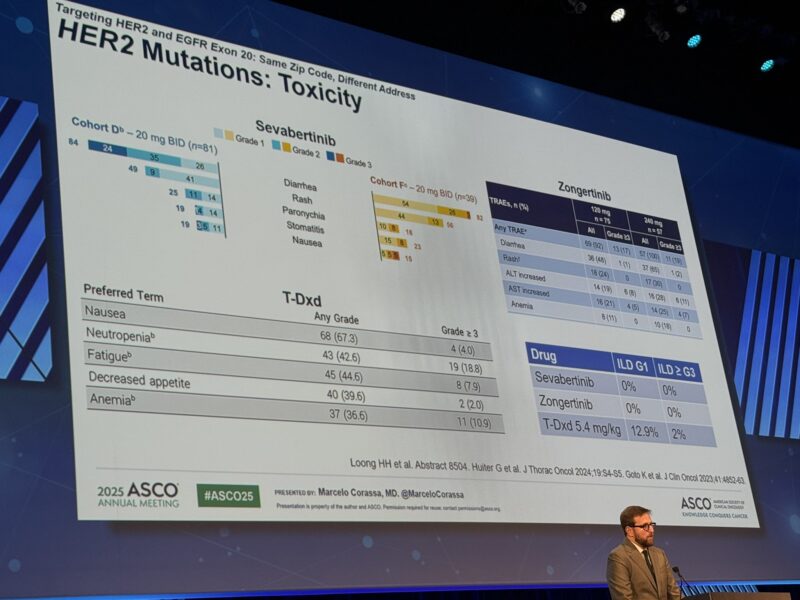
Treatment-related adverse events (TRAEs): Observed in 96.7% of patients. Diarrhea was the most common TRAE, leading to dose reduction in 8.3% of patients. No patient discontinued BAY 2927088 due to diarrhea, and no interstitial lung disease was reported.
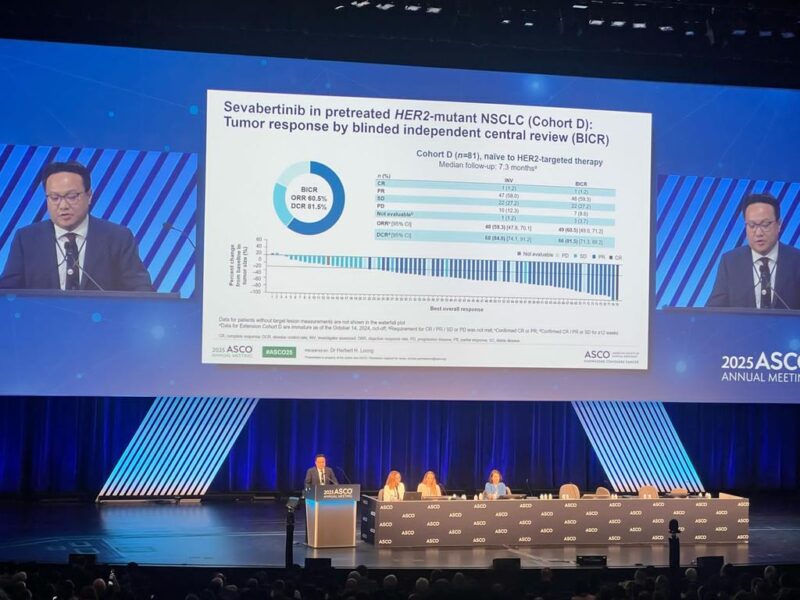
Objective response rate (ORR):
- Cohort D: 59.3% (95% CI 47.8–70.1)
- Cohort F: 59.0% (95% CI 42.1–74.4)
Disease control rate (DCR, confirmed response or stable disease ≥12 weeks):
- Cohort D: 84.0% (95% CI 74.1–91.2)
- Cohort F: 84.6% (95% CI 69.5–94.1)
One patient in Cohort D achieved a complete response.
Adverse Events Summary
Adverse Event Cohort D All Grades (%) Cohort D Grade ≥3 (%) Cohort F All Grades (%) Cohort F Grade ≥3 (%)
Any TRAE 96.3 38.3 97.4 20.5
Diarrhea 84.0 23.5 82.1 2.6
Rash 49.4 0 56.4 0
Paronychia 24.7 0 17.9 0
Stomatitis 18.5 1.2 23.1 0
What Are Specialists Saying About the SOHO-01 Trial?
SOHO-01 update: Phase I/II BAY 2927088 (Sevabertinib) HER2+ aNSCLC, Promising ORR 60% in both 1L and pretreated/HER2-naïve cohorts. Similar DoR ~9 mo. Most common AE diarrhea (all Gr 84%, ≥3 23%). Phase III SOHO-02 ongoing. Nicely discussed.
From Dr. Noemi Reguart’ X
Oral, Mets, SOHO-01: BAY 2927088 in pts with advanced HER2-mutant NSCLC who were pretreated but naïve to HER2-targeted Tx or had not received any Tx,ORR 59.3%/59.%, DOR ≥12wks 84.0%/84.6%
From Dr. Hidehito HORINOUCHI’s X
At #ASCO25, Dr. Herbert Loong presented results from SOHO-01 evaluating sevaberitinib in advanced HER2-mutant #NSCLC.In pretreated patients (Cohort D), confirmed ORR was 60.5%, with consistent activity across key subgroups including brain metastases and TKD mutations (ORR 65.3%). In first-line patients (Cohort F), early data showed ORR 59.0% and a DCR of 84.6%, despite shorter follow-up. Safety profile was manageable, with no ILD or pneumonitis reported. Only 1 grade 3 diarrhea case in the 1L cohort. A randomized Phase 3 study (SOHO-02, NCT06452277) is now underway to evaluate sevaberitinib in the 1L setting. Promising signs for expanding HER2-targeted strategies in lung cancer.
From Abner Antonio Murray’s X
Conclusions
BAY 2927088 demonstrated manageable safety and promising anti-tumor activity in both pretreated and treatment-naïve patients with advanced HER2-mutant NSCLC. Diarrhea was the most common adverse event but was manageable and did not lead to treatment discontinuation. Similar response rates were observed in both cohorts, supporting further development of BAY 2927088 in this population.

You Can Also Read BREAKWATER Trial Results: New Standard for BRAF V600E Metastatic Colorectal Cancer at ASCO 2025 by Oncodaily
Authors:
Herbert H. Loong, Lin Li, Lin Wu, Tae Min Kim, Arsela Prelaj, Xiaorong Dong, Hye Ryun Kim, Tsung-Ying Yang, Gennaro Daniele, Shun Lu, Yong Fang, Yuki Shinno, Liyun Miao, Nicolas Girard, Jun Zhao, Gerrina Ruiter, Virginie Aris, Rui Li, Paolo Grassi, Xiuning Le
Affiliations:
The Chinese University of Hong Kong, Hong Kong SAR, China; Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China; Hunan Cancer Hospital, Central South University, Changsha, China; Seoul National University Hospital, Seoul, South Korea; Fondazione IRCCS Istituto Nazionale dei Tumori di Milano, Milan, Italy; Huazhong University of Science and Technology, Wuhan, China; Yonsei Cancer Center, Seoul, South Korea; Taichung Veterans General Hospital, Taiwan; Fondazione Policlinico Universitario Agostino Gemelli, Rome, Italy; Shanghai Jiaotong University, Shanghai, China; Zhejiang University School of Medicine, Hangzhou, China;
National Cancer Center Hospital, Tokyo, Japan; Nanjing Drum Tower Hospital, Nanjing, China; Institut Curie, Paris, France; Peking University Cancer Hospital & Institute, Beijing, China; Netherlands Cancer Institute, Amsterdam, Netherlands; Bayer HealthCare Pharmaceuticals, Whippany, NJ; Bayer S.p.A., Milan, Italy; The University of Texas MD Anderson Cancer Center, Houston, TX.
Written by Aharon Tsaturyan MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
