STELLAR-002 Trial at ASCO 2025 Shows Promising Activity of Zanzalintinib Plus Nivolumab in Advanced Clear Cell Renal Cell Carcinoma
Dr. Jad Chahoud from Moffitt Cancer Center presented the results of the STELLAR-002 trial at ASCO 2025, shedding new light on the combination of zanza (XL092) with nivolumab (nivo), with or without relatlimab (rela), in patients with previously untreated advanced clear cell renal cell carcinoma (ccRCC). This article summarizes the key findings from this phase 1b study, focusing on the efficacy, safety, and clinical implications of this novel combination therapy for first-line treatment of metastatic ccRCC.
STELLAR-002 Trial Background
Clear cell renal cell carcinoma (ccRCC) is commonly treated with a combination of vascular endothelial growth factor receptor (VEGFR)-targeted tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) as the current standard of care. Zanza is a new oral multi-targeted TKI that inhibits VEGFR, MET, and TAM kinases (TYRO3, AXL, MER) and has a short half-life, which may improve its therapeutic index. Previous phase 1 data (STELLAR-001) showed zanza to be tolerable with antitumor activity in pre-treated advanced ccRCC patients.
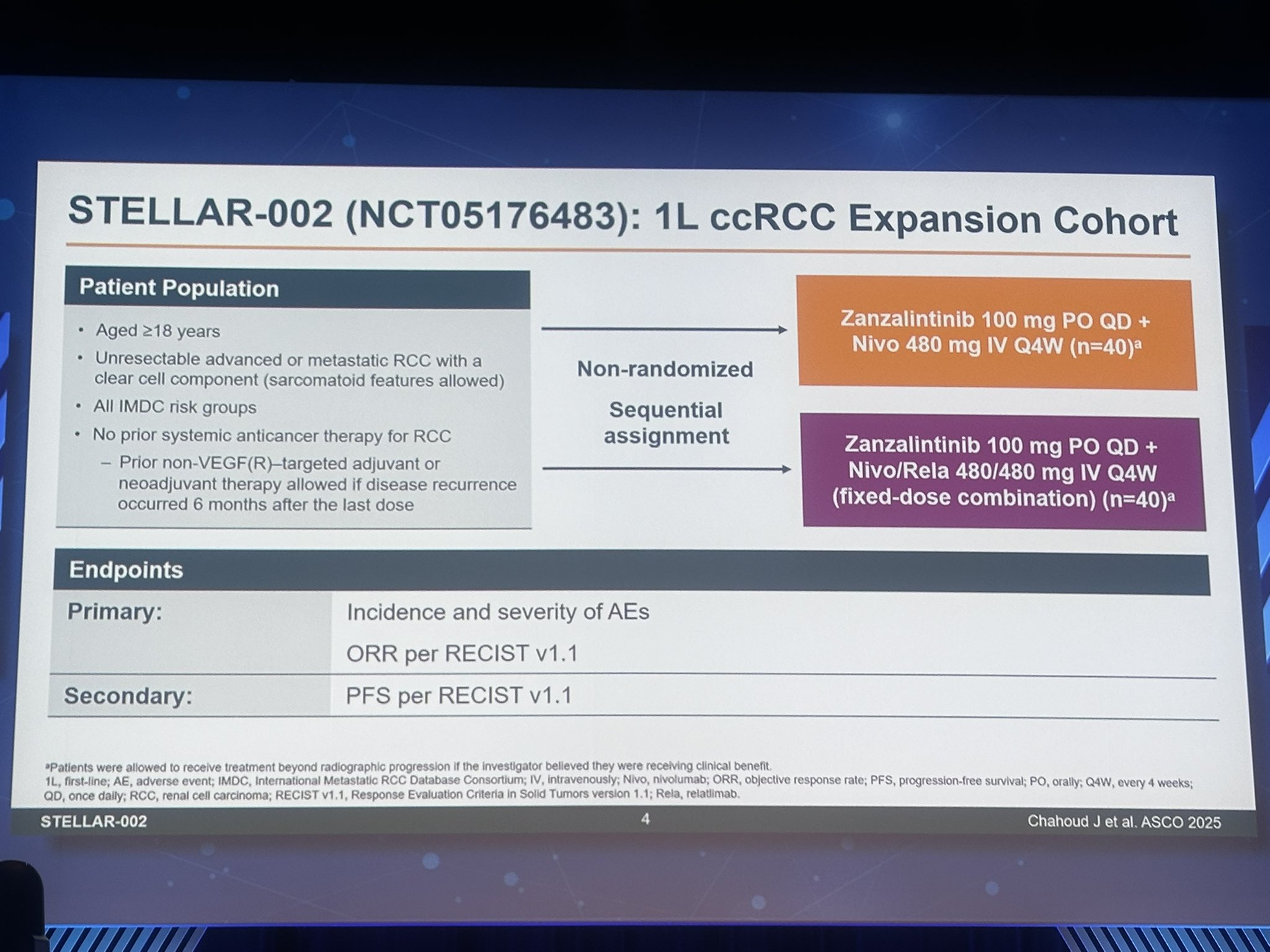
STELLAR-002 is a phase 1b, open-label study investigating the safety and efficacy of zanza combined with nivolumab or the fixed-dose combination of nivolumab and relatlimab in patients with previously untreated advanced or metastatic ccRCC.
Study Methods and Design
The Patient Population was adults with unresectable advanced or metastatic ccRCC, any International Metastatic RCC Database Consortium (IMDC) risk group, and no prior systemic therapy for advanced disease.
Treatment Arms
- Zanza 100 mg orally + nivolumab 480 mg IV every 4 weeks (q4w).
- Zanza 100 mg orally + nivolumab/relatlimab 480/480 mg IV q4w.
Endpoints
- Primary: Objective response rate (ORR) assessed by investigators per RECIST 1.1.
- Safety and tolerability profiles.
Key Results
Median follow-up was 16.1 months for zanza + nivo arm and 11.9 months for zanza + nivo/rela.
In the zanza plus nivolumab arm (n=40)
- ORR was 63%, including 4 complete responses (CRs) and 21 partial responses (PRs).
- Disease control rate (DCR: CR+PR+stable disease) reached 90%.
- Progression-free survival (PFS) rates were 83.2% at 6 months and 64.2% at 12 months.
- Most common treatment-emergent adverse events (TEAEs) were diarrhea (78%), hypertension (58%), and nausea (58%).
- Grade 3/4 adverse events related to zanza were primarily hypertension (30%) and diarrhea (15%).
- Treatment-related palmar-plantar erythrodysesthesia (PPE) occurred in 28% with 8% grade 3 severity, no grade 4.
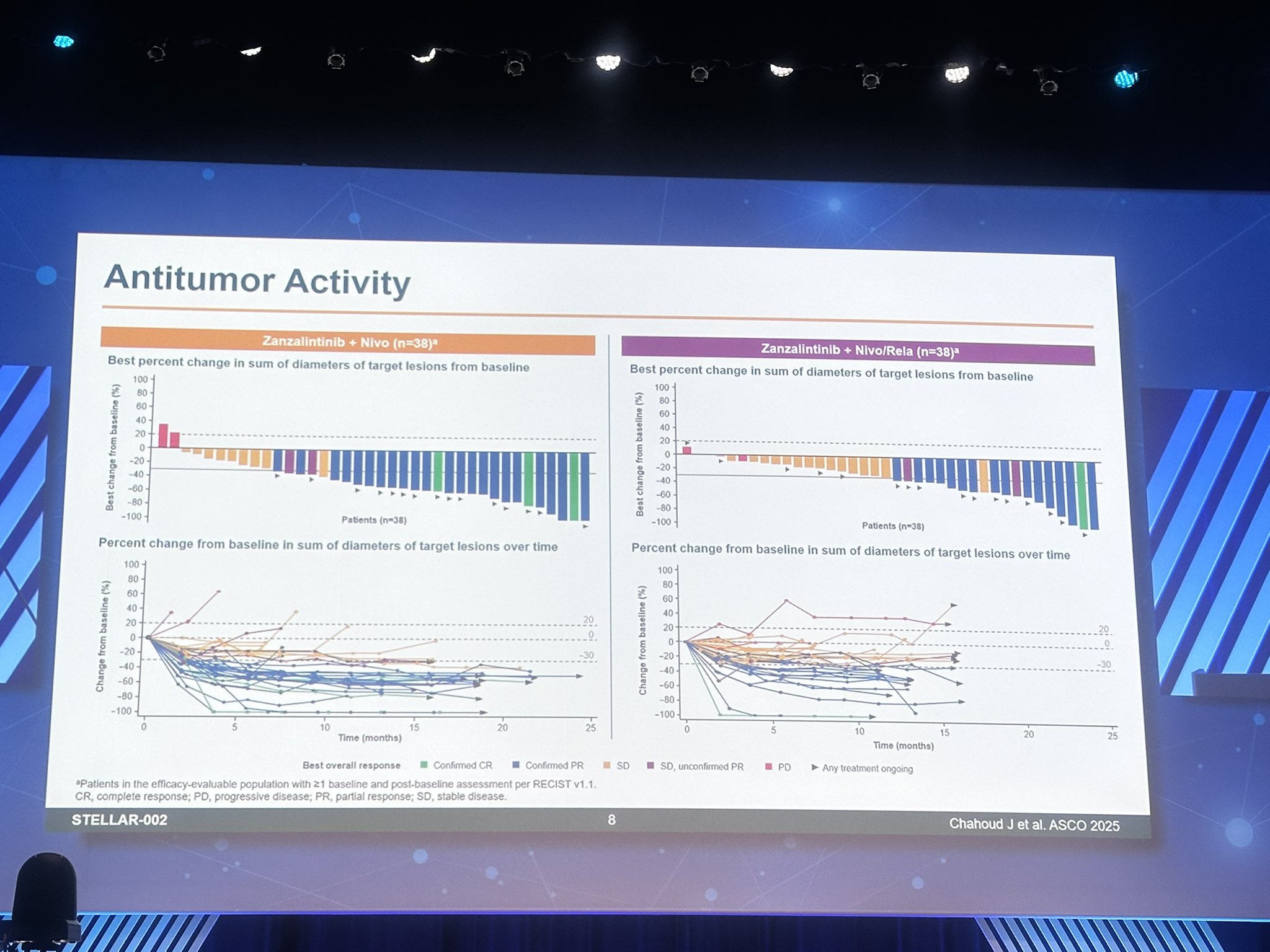
In the zanza plus nivolumab/relatlimab arm (n=40):
- ORR was 33%, with 1 CR and 12 PRs.
- DCR remained high at 90%.
- PFS rates were 80.2% at 6 months and 58.8% at 12 months.
- TEAEs included diarrhea (60%) and nausea (50%).
- Grade 3/4 hypertension related to zanza occurred in 13%.
- PPE was less frequent (5%) and no grade 3/4 PPE was reported.
- Seven patients (18%) discontinued treatment due to adverse events.
- No treatment-related deaths (grade 5 adverse events) were observed.
Key Takeaways
• The combination of zanza plus nivolumab demonstrated promising antitumor activity as first-line therapy in metastatic ccRCC, with an ORR of 63% and a manageable safety profile.
• Zanza’s short half-life may contribute to a favorable toxicity profile, including a relatively low rate of severe palmar-plantar erythrodysesthesia.
• The addition of relatlimab to nivolumab and zanza showed lower response rates but maintained high disease control, suggesting further investigation is needed to define its role.
• Hypertension and diarrhea remain the most frequent adverse effects and require careful management in clinical practice.
• These findings support further clinical development of zanza combinations as potential new standards for previously untreated advanced ccRCC.
This ASCO 2025 update on the STELLAR-002 trial highlights Zanza’s potential as a novel VEGFR/MET/TAM kinase inhibitor combined with immune checkpoint blockade to improve outcomes for patients with advanced clear cell renal cell carcinoma. Continued research will clarify optimal regimens and long-term benefits.
You Can Read the Original Abstract on ASCO
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023