Dostarlimab (Jemperli) 2025 Updates: Uses in Cancer, Side Effects, Dosage, Expectations, and More
Dostarlimab, sold under the brand name Jemperli, is a type of cancer immunotherapy known as a PD-1 inhibitor. It works by helping the body’s immune system recognize and attack cancer cells more effectively. Unlike traditional chemotherapy, which directly targets cancer cells, dostarlimab boosts the immune system to do the work. This approach can lead to long-lasting responses in some patients, especially those whose tumors have specific genetic features like mismatch repair deficiency (dMMR). This article explains how dostarlimab works, its side effects, dosage, and what to expect during treatment.
Which Company produced Dostarlimab?
Dostarlimab (brand name Jemperli) was discovered by AnaptysBio, a U.S.-based biotechnology company specializing in antibody therapies. In 2014, AnaptysBio licensed the drug to TESARO, Inc., which was later acquired by GSK (GlaxoSmithKline) in 2019. GSK is a global pharmaceutical company headquartered in the United Kingdom, known for its focus on research and development of innovative medicines and vaccines. Following the acquisition, GSK has been responsible for the ongoing research, development, manufacturing, and commercialization of dostarlimab.
In return, AnaptysBio retained the rights to future royalties and milestones, which it subsequently monetized through two royalty transactions with Sagard Healthcare Royalty Partners in 2021 and 2024, selling its entitlement to milestone payments and future royalties on Jemperli (dostarlimab).
How does Dostarlimab work?
Dostarlimab is a type of cancer immunotherapy known as a PD-1 (programmed death-1) checkpoint inhibitor. It works by helping the immune system recognize and attack cancer cells. Normally, the PD-1 receptor on immune cells binds to PD-L1 or PD-L2 proteins found on some normal and cancerous cells. This interaction acts as a “brake” to prevent the immune system from attacking. However, many tumors exploit this pathway to avoid detection.
By blocking PD-1 from binding to PD-L1 and PD-L2, dostarlimab releases this immune system brake, allowing T cells to stay active and target cancer cells more effectively. This is particularly helpful in cancers with deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H)—tumors that tend to have many mutations, making them more visible to the immune system.
This immune-based approach differs from traditional chemotherapy, as it doesn’t attack the tumor directly but instead helps the body’s own defenses fight the cancer.
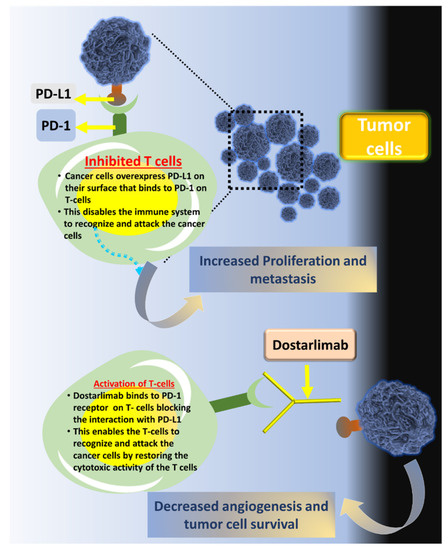
Photo from MDPI official website
What Cancers is Dostarlimab approved to treat?
Dostarlimab is approved by the U.S. Food and Drug Administration (FDA) to treat several types of advanced or recurrent cancers, especially those with deficient mismatch repair (dMMR) or high microsatellite instability (MSI-H).
In April 2021, dostarlimab first received FDA approval for women with recurrent or advanced dMMR endometrial cancer that had progressed following prior treatment. This was followed by an accelerated approval in August 2021, expanding its use to adult patients with dMMR advanced or recurrent solid tumors, including those with no satisfactory alternative treatment options.
By February 2023, the FDA granted regular approval for dostarlimab as a single agent for treating recurrent or advanced dMMR endometrial cancer. In July 2023, dostarlimab combined with chemotherapy was approved for patients with dMMR or MSI-H primary advanced or recurrent endometrial cancer. Most recently, in August 2024, the FDA expanded this combination approval to include all adult patients with primary advanced or recurrent endometrial cancer, regardless of biomarker status. These approvals highlight dostarlimab’s growing role in treating difficult-to-treat cancers, particularly those involving DNA repair deficiencies.
You can read more about Endometrioid Adenocarcinoma on OncoDaily.
What research is behind the approval?
Dostarlimab’s accelerated approval was based on results from the GARNET trial (cohort A1), which studied 71 patients with mismatch repair–deficient (dMMR) advanced or recurrent endometrial cancer that progressed after platinum-based chemotherapy. Patients received 500 mg IV every 3 weeks for 4 doses, followed by 1,000 mg IV every 6 weeks.
The overall response rate (ORR) was 42.3%, including 12.7% complete responses and 29.6% partial responses. Median duration of response (DOR) was not reached, with 93.3% of responders maintaining benefit for at least 6 months (range: 2.6–22.4+ months).
Serious side effects occurred in 34% of patients, including infections, kidney injury, and abdominal pain. Common side effects were fatigue, nausea, diarrhea, anemia, and constipation. Some patients experienced immune-related reactions like pneumonitis, colitis, or hepatitis.
Find more information about this approval on FDA official website.

Learn more about Stomach Cancer: Symptoms and Causes, Types, Diagnosis and Treatment on OncoDaily.
A 2021 Journal of Clinical Oncology study from the GARNET trial (NCT02715284) evaluated dostarlimab in patients with mismatch repair–deficient (dMMR) or microsatellite instability–high (MSI-H) solid tumors. Among 209 patients, the overall response rate (ORR) was 41.6% (95% CI: 34.9–48.6), including 44.7% in endometrial cancer and 38.7% in non-endometrial tumors. The median duration of response was 34.7 months, with 95.4% maintaining response for ≥6 months.
Treatment-related side effects were mostly mild, with fatigue, diarrhea, and asthenia being the most common. Grade ≥3 events were rare, and no deaths were linked to treatment.
Learn more about Ovarian Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
Dostarlimab (Jemperli) received full FDA approval for dMMR advanced endometrial cancer based on GARNET trial data (141 patients). The overall response rate was 45.4%, with 15.6% complete and 29.8% partial responses. Most responses lasted ≥12 months (85.9%), and over half ≥24 months (54.7%).
Common side effects include fatigue, anemia, rash, nausea, and diarrhea. The recommended dose is 500 mg IV every 3 weeks (4 doses), then 1,000 mg every 6 weeks.
The RUBY/ENGOT-EN6/GOG3031/NSGO phase 3 trial published in The New England Journal of Medicine (2023) showed that adding dostarlimab to carboplatin–paclitaxel significantly improved outcomes in advanced or recurrent endometrial cancer. Among 494 patients, 118 had dMMR/MSI-H tumors. At 24 months, progression-free survival (PFS) in this group was 61.4% with dostarlimab versus 15.7% with placebo (HR 0.28; P<0.001). In the overall population, PFS was 36.1% vs. 18.1% (HR 0.64; P<0.001), and overall survival at 24 months was 71.3% with dostarlimab vs. 56.0% with placebo (HR 0.64).
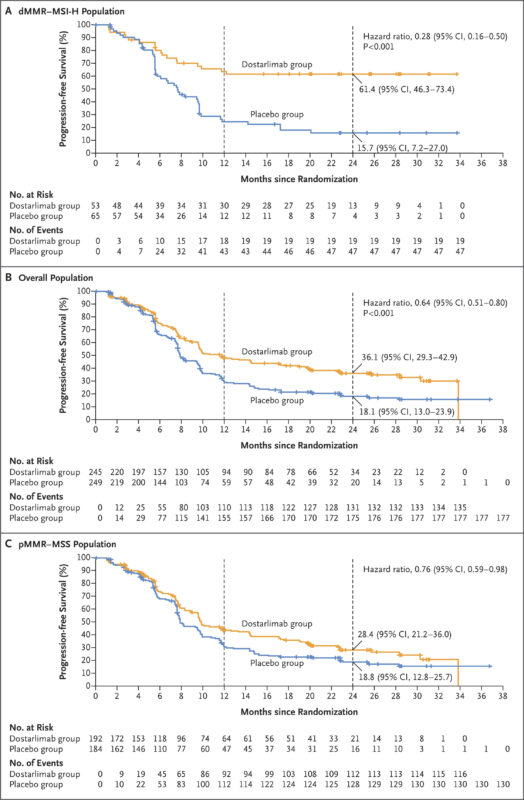
A later update, published on the FDA’s official website, provided long-term outcome data, showing a median overall survival of 44.6 months with dostarlimab versus 28.2 months with placebo (HR: 0.69). Median progression-free survival was also longer—11.8 months compared to 7.9 months (HR: 0.64).
The most common side effects in the updated analysis included anemia, fatigue, neuropathy, nausea, and elevated liver enzymes. The safety profile remained consistent with the known effects of the individual agents. Dostarlimab was administered at 500 mg every 3 weeks during chemotherapy, followed by 1,000 mg every 6 weeks as monotherapy.
Dostarlimab side effects and its management
Dostarlimab (brand name Jemperli) is an immunotherapy used to treat certain advanced cancers. While it offers therapeutic benefits, it can also cause side effects that vary in severity. Understanding these side effects and their management is crucial for patients undergoing treatment.
Common side effects
Some side effects occur more frequently among patients taking dostarlimab. One of the most common is fatigue, which can affect a patient’s daily activities and overall well-being. Gastrointestinal issues such as diarrhea, constipation, nausea, and vomiting are also frequently reported. These symptoms can often be controlled with dietary adjustments and medications, but they should be discussed with a healthcare provider if they persist or worsen.
Anemia, or low red blood cell count, may develop in some patients, leading to symptoms such as tiredness, shortness of breath, and pale skin. Headaches, fever, decreased appetite, and joint or muscle pain are additional side effects that may occur during treatment. Skin reactions like rash or itching are also seen in some individuals and are typically mild, though they can sometimes become more bothersome.
Less Common Side Effects
Less frequently, patients may experience more unusual symptoms such as blurred vision, dizziness, or dry eyes. Immune-related side effects can also develop, as dostarlimab activates the immune system. These may include inflammation in different organs, such as the lungs (pneumonitis), liver (hepatitis), intestines (colitis), or thyroid gland (thyroiditis or hypothyroidism). Though these conditions are less common, they can be serious if not detected and treated promptly.
In rare cases, dostarlimab may affect the kidneys or pancreas or cause hormone imbalances that impact metabolism and energy levels. Some patients may also notice changes in liver enzymes during blood tests, even without obvious symptoms. It is important for patients to undergo regular lab monitoring to catch these issues early.
Management of Side Effects
Managing side effects involves regular monitoring and supportive care tailored to each symptom. For instance, fatigue can be addressed through rest and activity adjustments, while gastrointestinal symptoms like nausea and diarrhea may be managed with dietary changes and medications. It’s essential for patients to maintain open communication with their healthcare team to effectively manage any side effects that arise during treatment.

What is the Recommended Dosage of Dostarlimab?
For most patients with mismatch repair-deficient (dMMR) tumors, the usual dose is 500 mg every 3 weeks for the first four doses, followed by 1,000 mg every 6 weeks. In endometrial cancer, dostarlimab can be used alone or with chemotherapy, depending on the treatment plan.Dose reductions are generally not recommended. However, treatment may be paused or stopped if serious side effects occur.
How is Dostarlimab administered?
Dostarlimab is given as an intravenous (IV) infusion and should never be mixed with other drugs in the same infusion line. It is prepared by diluting the medicine into a bag of saline (0.9% sodium chloride) or 5% dextrose solution before administration.
Before use, the vial is checked to ensure the liquid is clear or slightly yellow without any particles. The medicine should be gently mixed by inverting the bag but never shaken. The infusion takes about 30 minutes and is given through specific tubing with a fine filter to ensure safety.
Dostarlimab should not be given as a quick injection (IV push) or mixed with other medications in the same line. Unopened vials are stored in the refrigerator between 2-8°C and protected from light. Once prepared, the diluted solution can be kept at room temperature for up to 6 hours or refrigerated for up to 24 hours but should not be frozen.
How Is the Drug Metabolized and Eliminated from the Body?
The drug is gradually broken down into smaller peptides and amino acids through the body’s natural catabolic pathways. It stays in the body for quite a while, with a half-life of about 25 days, and is cleared slowly at a rate of 0.007 liters per hour.
What to Avoid During Dostarlimab Treatment?
During treatment with dostarlimab, it is important to avoid certain things to reduce the risk of side effects and ensure the therapy works effectively. Patients should avoid receiving live vaccines, such as the measles, mumps, and rubella (MMR) vaccine or the live flu vaccine, because dostarlimab affects the immune system and could increase the risk of infection.
Patients should also avoid taking other immunosuppressive drugs or steroids unless prescribed by their doctor, as these can interfere with dostarlimab’s activity and may increase the risk of serious infections or reduce the treatment’s effectiveness.
Alcohol consumption should be limited or avoided, as it can worsen some side effects like fatigue, nausea, or liver problems. It is important to inform the healthcare provider about all medications, supplements, or herbal products being taken to avoid harmful interactions.
Dostarlimab’s effectiveness over time
Dostarlimab has shown durable effectiveness in endometrial cancer, with many patients experiencing long-lasting responses. In the GARNET trial, over 85% of responders maintained their response for at least 12 months, and more than half for over 24 months. In the RUBY trial, it significantly prolonged both progression-free and overall survival, with benefits sustained over a median follow-up of more than three years.
Ongoing trials with Dostarlimab
Two promising clinical trials are currently investigating dostarlimab, a PD-1 inhibitor, in solid tumors. The PULMO-01 trial (NCT06879717) is evaluating dostarlimab in combination with roginolisib, with or without docetaxel, in patients with advanced non-squamous non-small cell lung cancer (NSCLC) who have progressed after standard therapies. The study aims to assess safety and the effect on immune-suppressing Treg cells.
Meanwhile, the Superhero trial (NCT06830239) is testing neoadjuvant dostarlimab in patients with resectable stage III or T3–T4 pMMR/MSS colon cancer. Administered before surgery, the goal is to observe molecular changes in tumors and evaluate potential benefits of this short-course immunotherapy. These trials highlight ongoing efforts to expand dostarlimab’s role beyond its current approvals.
FAQ
What is Dostarlimab?
A PD‑1 inhibitor immunotherapy (brand name Jemperli) that unleashes the immune system to attack cancer cells, especially in dMMR/MSI‑H tumors.
Which cancers does Dostarlimab treat?
FDA‑approved for advanced or recurrent endometrial cancer and other dMMR/MSI‑H solid tumors, alone or with chemotherapy.
What are common side effects of Dostarlimab?
Common side effects include fatigue, anemia, nausea, rash, and diarrhea. Immune-related adverse events like pneumonitis, colitis, and hepatitis can also occur but are typically manageable with monitoring and supportive care.
What is the recommended dosage and administration schedule?
500 mg IV every 3 weeks for four doses, then 1,000 mg IV every 6 weeks, with or without chemotherapy.
How is Dostarlimab metabolized?
Dostarlimab is broken down into peptides and amino acids via catabolic pathways. It has a long half-life of around 25 days and is slowly cleared from the body.
What precautions should patients take during Dostarlimab treatment?
Patients should avoid live vaccines and consult their doctor before using steroids or immunosuppressants. Alcohol intake should be limited, and all supplements and medications should be reviewed for interactions.
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
